[Mr_GreenCoat] is studying engineering. His thermodynamics teacher agreed with the stance that engineering is best learned through experimentation, and tasked [Mr_GreenCoat]’s group with the construction of a vacuum chamber to prove that the boiling point of a liquid goes down with the pressure it is exposed to.
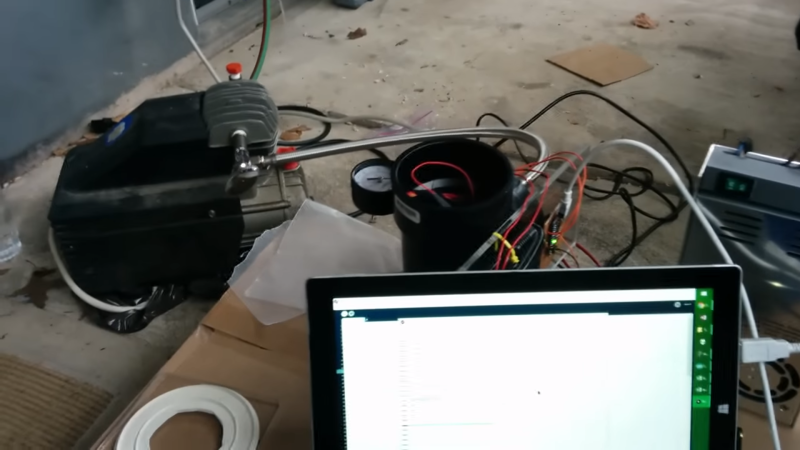
His group used black PVC pipe to construct their chamber. They used an air compressor to generate the vacuum. The lid is a sheet of lexan with a silicone disk. We’ve covered these sorts of designs before. Since a vacuum chamber is at max going to suffer 14.9 ish psi distributed load on the outside there’s no real worry of their design going too horribly wrong.
The interesting part of the build is the hardware and software built to boil the water and log the temperatures and pressures. Science isn’t done until something is written down after all. They have a power resistor and a temperature probe inside of the chamber. The temperature over time is logged using an Arduino and a bit of processing code.
In the end their experiment matched what they had been learning in class. The current laws of thermodynamics are still in effect — all is right in the universe — and these poor students can probably save some money and get along with an old edition of the textbook. Video after the break.















Nice demo.
First!
Lame!
So, who else felt a shiver run up their spine when the read that PVC was being used as a pressure vessel.
It should be alright. It’s going to be max 16-ish psi on the outside of a cylindrical vessel with no chips or cracks on it.
http://www.engineeringtoolbox.com/pvc-cpvc-pipes-pressures-d_796.html
No shiver, used to use PVC vacuum chamber wrapped with a heat pad to dry samples, then I graduated to a pressure cooker and hot plate.
It’s not being used as a pressure vessel, it’s being used as a vacuum vessel.
PVC is acceptable for use as both a pressure and a vacuum vessel, as indicated by pressure and collapse ratings.
http://www.lascofittings.com/pressureratingsofplastics
PVC isn’t acceptable for this application. One problem is that PVC turns into shrapnel when it fails. Also, the oils typically used in air compressors (and vacuum pumps) cause degradation of the piping over time. PVC will fail a OSHA inspection when used for compressed air.
Use something that stretches/crushes and remains mostly intact when it bursts, and doesn’t degrade from the oils used in the pump/compressor.
Using the browser word search didn’t the words air or gas. The reference deal with fluids only. Many metal fitting have two pressure rating ratings on for fluid another for steam. The maximum pressure under steam is lower than for fluid. Of course PVC isn’t going to handle steam, but I have never seen any plastic pipe imprinted with maximum pressure rating for gas.
Gas is a fluid. Steam is a hot fluid.
“… to prove that the boiling point of a liquid goes down with the pressure it is exposed to.” Huh? I was taught in physics class too many decades ago that the boiling point (temperature) of a liquid goes UP with pressure (and down with a reduction in pressure). That is why water boils at room temperature in a partial vacuum (below the vapor pressure of water). Has somebody gone and changed the laws of physics since I attended University? Or is that a typographical error?
Donald trump changed the laws of pisics
Good thing he didn’t change the laws of physics, because we would really be in trouble then.
So that’s how his wall is cost effective!
Ahhh, so he’s going to hack Mexico’s bank account to get the money to pay for it?
…I guess that makes sense.
a lower pressure is still a pressure. sentence is true but confusing.
The wording suggests an (untrue) inversely proportional relative pressure CHANGE. Accurate language is important when this forum attracts geeks (most of whom have some degree of Asperger’s Syndrome, where word precision and accurace is the major key to understanding). We are all at least a little like Sheldon Cooper (Big Bang Theory TV show) or we would not be here, right? Or better yet, think Spock (that does not compute!)… ;-)
If you have to get everything spelled out for you and pre-chewed, maybe you are farther from a ‘Sheldon Cooper’ than you think.
I suspect that most of us here knew precisely what the author meant.
Or more simply, the original choice of words in the first post above suggests that the boiling point varies INVERSELY (goes down) with pressure, which is incorrect.
https://en.wikipedia.org/wiki/Vapor_pressure
“The atmospheric pressure boiling point of a liquid (also known as the normal boiling point) is the temperature at which the vapor pressure equals the ambient atmospheric pressure. With any incremental increase in that temperature, the vapor pressure becomes sufficient to overcome atmospheric pressure and lift the liquid to form vapor bubbles inside the bulk of the substance.”
With as in along with. Ya dang pedants. Stealin’ all these here funs I’m havin’ with these writin’s I like to scribble.
To me that reads that as the pressure goes down, the boiling point goes down with it. Low pressure is still pressure.
They are implying that it goes down along with the pressure. Both are decreasing, not at the same rate mind you, but going down nonetheless.
But yeah, wording aside, I love DIY projects, and now I want a vacuum chamber too. Though in my case, I want it to do some metal sputtering…
+1
Ion deposition is a pretty handy skill. Anyone done anything reasonable to do that?
https://www.youtube.com/watch?v=c4Sic1DRXJI
https://www.youtube.com/watch?v=TV5p36-c3R4
https://www.youtube.com/watch?v=9OEz_e9C4KM
Thanks for the links!
These students need to invest in a camera tripod.
I do this demonstration in class, though I use a bell jar and borrow a student’s uncapped water bottle (be sure it’s single-wall so it doesn’t burst). When I give it back to them colder than before, the whole class “gets it” in a way that might have taken a whole lecture or two otherwise…kind of a turning point for the concept of enthalpy/vapor pressure etc.
It doesn’t hurt that this is usually before the spring thaw so we have steam condensing in the baseboard air handlers to show how that enthalpy tied up in vapor can be used somewhere else.
When I then link that to vacuum cooling of produce they understand why we can have fresh lettuce in the dead of winter.
http://www.southernvacuumcooling.com/Vacuum%20Cooling.htm
it is not my experience that any more than the top few percent of undergraduates “get” anything about thermodynamics.
The pressure rating of PVC is terrific – just ask any kid that’s made potatoe cannon.
Just don’t ask about the ones that failed ;-)