[Chase’s] post titled “How to Grow Sodium Chloride Crystals at Home” might as well be called “Everything You Always Wanted to Know about Salt Crystals (but Were Afraid to Ask).” We aren’t sure what the purpose of having transparent NaCl crystals are, but we have to admit, they look awfully cool.
Sodium chloride, of course, is just ordinary table salt. If the post were simply about growing random ugly crystals, we’d probably have passed over it. But these crystals — some of them pretty large — look like artisan pieces of glasswork. [Chase] reports that growing crystals looks easy, but growing attractive crystals can be hard because of temperature, dust, and other factors.
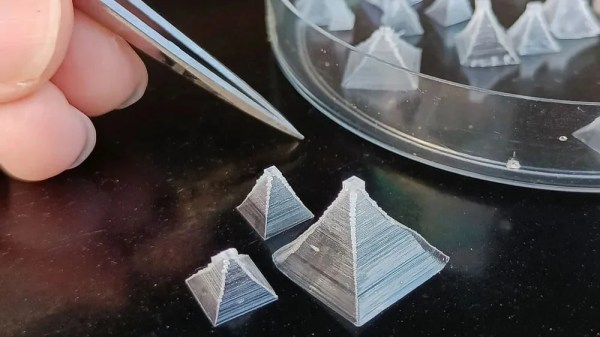
You probably have most of what you need. Table salt that doesn’t include iodine, a post, a spoon, a funnel, filter paper, and some containers. You’ll probably want tweezers, too. The cooling rate seems to be very important. There are pictures of what perfect seed crystals look like and what happens when the crystals form too fast. Quite a difference! Once you find a perfectly square and transparent seed crystal, you can use it to make bigger ones.
After the initial instructions, there is roughly half the post devoted to topics like the effect growth rate has on the crystal along with many pictures. There are also notes on how to form the crystals into interesting shapes like stars and pyramids. You can also see what happens if you use iodized salt.
If salt is too tame for you, try tin. Or opt for copper, if you prefer that.