For all of the semiconductor industry’s legendary reputation for cleanliness, the actual processes that go into making chips use some of the nastiest stuff imaginable. Silicon oxide is comes from nothing but boring old sand, and once it’s turned into ultrapure crystals and sliced into wafers, it still doesn’t do much. Making it into working circuits requires dopants like phosphorous and boron to give the silicon the proper semiconductor properties. But even then, a doped wafer doesn’t do much until an insulating layer of silicon dioxide is added and the unwanted bits are etched away. That’s a tall order, though; silicon dioxide is notoriously tough stuff, largely unreactive and therefore resistant to most chemicals. Only one substance will do the job: hydrofluoric acid, or HFA.
HFA has a bad reputation, and deservedly so, notwithstanding its somewhat overwrought treatment by Hollywood. It’s corrosive to just about everything, it’s extremely toxic, and if enough of it gets on your skin it’ll kill you slowly and leave you in agony the entire time. But it’s also absolutely necessary to make everything from pharmaceuticals to cookware, and it takes some big chemistry to do it safely and cheaply.

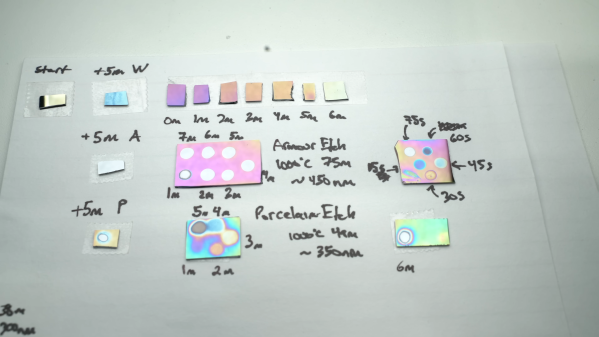


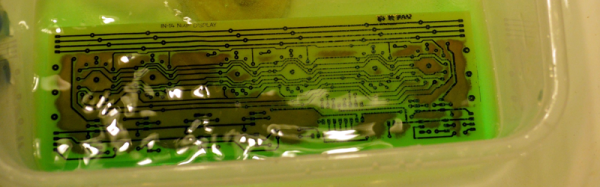
 In the interest of the scientific method [Feynmaniac] (great name, btw) over on Instructables
In the interest of the scientific method [Feynmaniac] (great name, btw) over on Instructables 








