We’ve all seen recreations of the famous double-slit experiment, which showed that light can behave both as a wave and as a particle. Or rather, it’s likely that what we’ve seen is the results of the double-slit experiment, that barcode-looking pattern of light and dark stripes, accompanied by some handwaving about classical versus quantum mechanics. But if you’ve got 20 minutes to invest, this video of the whole double-slit experiment cuts through the handwaving and opens your eyes to the quantum world.
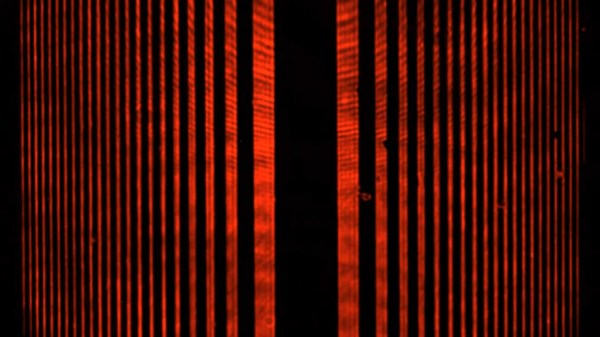
For anyone unfamiliar with the double-slit experiment, [Huygens Optics] actually doesn’t spend that much time explaining the background. Our explainer does a great job on the topic, but suffice it to say that when coherent light passes through two closely spaced, extremely fine openings, a characteristic pattern of alternating light and dark bands can be observed. On the one hand, this demonstrates the wave nature of light, just as waves on the ocean or sound waves interfere constructively and destructively. On the other hand, the varying intensity across the interference pattern suggests a particle nature to light.
To resolve this conundrum, [Huygens] jumps right into the experiment, which he claims can be done with simple, easily sourced equipment. This is belied a little by the fact that he used photolithography to create his slits, but it should still be possible to reproduce with slits made in more traditional ways. The most fascinating bit of this for us was the demonstration of single-photon self-interference using nothing but neutral density filters and a CCD camera. The explanation that follows of how it can be that a single photon can pass through both slits at the same time is one of the most approachable expositions on quantum mechanics we’ve ever heard.
[Huygens Optics] has done some really fascinating stuff lately, from variable profile mirrors to precision spirit levels. This one, though, really helped scratch our quantum itch.