Anything related to nanotechnology feels fairly modern, doesn’t it? Although Richard Feynman planted the seeds of the idea in 1959, the word itself didn’t really get formed until the 70s or 80s, depending on who you ask. But there is evidence that nanotechnology could have existed as far back as the 4th century in ancient Rome.
That evidence lies in this, the Lycurgus cup. It’s an example of dichroic glass — that is, glass that takes on a different color depending on the light source. In this case, the opaque green of front lighting gives way to glowing red when light is shining through it. The mythology that explains the scene varies a bit, but the main character is King Lycurgus, king of Edoni in Thrace.
So how does it work? The glass contains extremely small quantities of colloidal gold and silver — nanoparticles of gold to produce the red, and silver particles to make the milky green. The composition of the Lycurgus cup was puzzling until the 1990s, when small pieces of the same type of glass were discovered in ancient Roman ruins and analyzed. The particles in the Lycurgus cup are thought to be the size of one thousandth of a grain of table salt — substantial enough to reflect light without blocking it.
The question is, how much did the Romans know about what they were doing? Did they really have the means to grind these particles into dust and purposely infuse them, or could this dichroic glass have been produced purely by accident? Be sure to check out the videos after the break that discuss this fascinating piece of drinkware.
Continue reading “Nanotechnology In Ancient Rome? There Is Evidence”


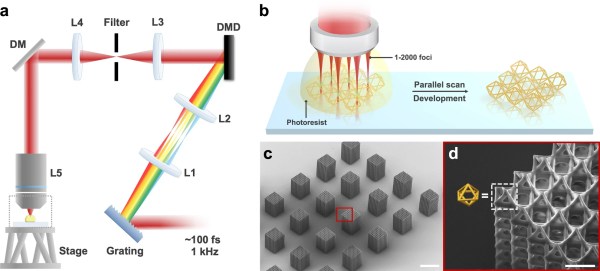
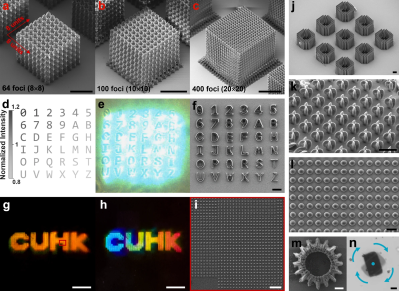 photopolymer resin, which is chemically tweaked to make it sensitive to the UV frequency photons. This is all fine, but as we know, this method is slow and can be of limited resolution, and has been largely superseded by LCD technology. Recent research has focussed on
photopolymer resin, which is chemically tweaked to make it sensitive to the UV frequency photons. This is all fine, but as we know, this method is slow and can be of limited resolution, and has been largely superseded by LCD technology. Recent research has focussed on 

 (
(