As the light of the 20th century was peeking over the horizon, a young physicist by the name of Max Planck was taking to heart some career advice he had received while he attended Munich University in Germany. With the recent discovery of thermodynamics, there wasn’t much left in physics to know, or so his adviser thought. Hindsight is indeed 20/20.
It turns out that Planck was an expert at thermodynamics. Having mastered the subject gave him some leverage to use against a growing group of physicists known as atomists who were using statistical models along with so called ‘atoms’ to predict experimental outcomes. Atomists believed that matter was composed of discrete units. Planck believed the world was continuous and could not be divided into any type of discrete component. And he would draw the second law of thermodynamics from his holster and put this atom idea in the clay.
Entropy to the Rescue
For the sake of argument, let us severely oversimplify the second law of thermodynamics to say that the entropy in an enclosed system will increase and it can’t be reversed. Entropy is the measure of disorder in a system. If we introduce gas into an enclosed container isolated from the outside world, the entropy of that gas would increase spontaneously to maximum, where it would be at equilibrium with its surroundings. And you can’t undo that process.

Now let us use this knowledge to formulate a plot to take out the atomists and their silly atom idea. Imagine we introduce two gasses, each at a different temperature, into a closed container. The 2nd law says that the temperatures would equilibrate as the entropy rises to its maximum value.
The atomists view is that this happens as a result of the mechanical interactions of the atoms comprising the gas. And that the equilibrium state is simply the most statistically probable state. If this were true, Planck would argue, then there is nothing to say that the process cannot be reversed. That the gas atoms could be separated and restored to their original temperatures and thus reducing the overall entropy of the system. Which is a direct contraction to the 2nd law of thermodynamics.
Ludwig Boltzmann, lead atomisit and thusly Planck’s arch rival, responded to the challenge with more statistics. He argued against the 2nd law itself, saying that the idea that entropy always increases is incorrect. Statistically speaking, it almost always increases. There is a chance, however small, that it could decrease. Basically he said that if you wait long enough, you could catch the entropy decreasing and the different gas atoms separating. The odds of such an event would be similar to the odds of a shattered light bulb reassembling itself. Mathematically, the time required works out to longer than the current age of the universe. While this might sound ludicrous, Boltzmann is on theoretical solid ground. His statistics had provided a proverbial bullet proof vest against Planck and his thermodynamics.
Black Body Radiation
This was the tipping point for Planck. Seeing his beloved thermodynamics stained with this ridiculous statistical hyperbole pushed him even harder to squash the atomists. He would turn to another issue in classical physics that was providing some headaches. It has to do with what is called cavity radiation. You see this in action whenever you turn on an incandescent light bulb. As a substance heats up, it will begin to emit light. The higher the temperature, the higher intensity and frequency of light emitted. Low temperatures, relatively speaking, will produce a dull red glow while the highest temperatures will produce a bright white light.
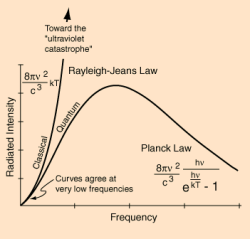
Physicists had simplified the process to what is called a black body. A black body is completely theoretical in concept. It absorbs all light radiation and is at a state of thermal equilibrium. That’s just fancy talk for saying the thing’s the same temperature throughout. The black body will emit radiation, called black body radiation, and the intensity of that radiation will be related to the body’s temperature. Now, if one sticks the thing in box a pokes a little hole in it that allows one to put radiation into the black body and then see what comes out, one can study the black body radiation in detail.
By doing black body experiments, physicists had discovered that the ratio of energy going in the little hole and energy coming back out was not dependent on the cavity shape or what it was made of. This led physicists to believe that there was something very, very fundamental to the nature of the radiation itself going on that they did not understand.
Physicist Wilhelm Wien had shown that there was a mathematical relationship between the temperature of the black body and the frequency of the resulting emitted radiation. But it only worked for higher frequencies. Two physicists by the names of Lord Rayleigh and Sir James Jeans would later go on to show how the output energy was related to frequency. Unlike Wien’s work, their equations accounted for experimental observations at the lower frequency ranges. But it didn’t work at higher frequencies. According to their math, as the frequency of emitted radiation tended toward the ultraviolet range, the output energy would have to be infinite, birthing the phrase Ultraviolet Catastrophe. Clearly something was missing.
Planck’s Change of Heart

Lest we forget about our Planck – Boltzmann feud, Planck had originally turned his attention to the ultraviolet catastrophe in order to show that nature was not composed of atoms, but was continuous throughout. It didn’t take him long to figure out how to mend Wien and Rayleigh-Jeans’ work into something that would agree with experiments at all frequency ranges. Through what was mostly guesswork, he came up with this little constant that made all the numbers add up. After doing some black body experiments, his equations appeared to work across the entire spectrum. Its significance would not be made apparent, however, until he was forced to come up with a theoretical basis for it, for which he would later say “…led to some weeks of the most strenuous work of my life”.
We’ll save the details on how Planck figured out where the constant that bears his name came from for another article, but let us understand for the moment that as he dug into the idea of this fundamental constant, the math kept taking him in the statistical direction…a direction he did not want to go. Boltzmann was able to account for entropy of a gas by assuming that energy was contained in the individual atoms. Planck was forced to consider the idea that entropy and probability were linked. This consideration led him to understand that, just as Boltzmann’s atoms of gas were little packets of energy, so too was his constant.
“We therefore regard – and this is the most essential part of the entire calculation – energy to be composed of a very definite number of equally finite packets.”
It would have been impossible for Max Planck to know that his energy packet idea, later to be called a quanta, would birth a new understanding of the very nature of reality itself.
Sources:
The Quantum Story, by Jim Baggott. Chapter 1 ISBN-978-0199566846














career advise?
http://blog.dictionary.com/advice-vs-advise/
Advice is always a noun and advise is always a verb.
Fixed. Thanks!
Often, the advice one gives as an advisor is invaluable.
“As the light of the 19th century was peeking over the horizon..” I think you mean 20th.
Damn it, I always do that!
He meant the other horizon, the Western one.
But you left all the crucial and interesting bits out, and simply handwaved it on the “frequencies” bit, and on how or why the whole problem was solved.
This article is just bad.
The whole point was that the cavity or the black body was thought to contain some sort of resonant oscillator, like a beer bottle that hums when you blow across it, that was collecting up energy and returning it back as radiation. Obviously the resonator can oscillate at any harmonic frequency that fits in the bottle, such as 1,2,4,8… 2^n times the fundamental frequency or the longest wavelength that fits in the cavity in the first place.
These resonant frequencies correspond to the spectrum or distribution of radiation wavelengths that come out of the black body, and the problem was in trying to explain why the energy comes out with a certain temperature-dependent spectrum, because the equipartition theorem suggested that there was nothing special in any of the frequencies and that all the energy should spread proportionally.
The issue is, that when you double the frequency you double the energy in that particular component of the spectrum. Each frequency can contain only a limited amount of energy, limited by the amplitude of the wave that can be contained in the cavity, so the energy has to be spread among all the frequencies, and as the energy contained in a wave increases as the frequency increases, nearly all the energy coming out of the black body should have been in the ultraviolet or beyond, which is empirically not the case.
Wikipedia explains it succintly as such:
“An example, from Mason’s A History of the Sciences,[2] illustrates multi-mode vibration via a piece of string. As a natural vibrator, the string will oscillate with specific modes (the standing waves of a string in harmonic resonance), dependent on the length of the string. In classical physics, a radiator of energy will act as a natural vibrator. And, since each mode will have the same energy, most of the energy in a natural vibrator will be in the smaller wavelengths and higher frequencies, where most of the modes are.”
In other words, according to classical mechanics, nearly all the energy you put in to a cavity or a black body should have come out as ultraviolet light of infinite frequency, and -that- is the ultraviolet catastrophe.
Too technical. I wanted to focus on the story of how Planck found his constant by bickering with Boltzmann. The Rayleigh-Jeans equation works for lower frequencies.
In the process you end up glossing over the whole topic of the article and just going “And then the guys did something brilliant and it was awesome; I’ll save you the details.”
+1. Narrative is fine, but it has to carry enough substance – in particular, the story is the background in front of which the science unfolds, not the other way around.
Saying “the Rayleigh-Jeans equation works for lower frequencies” is misleading, because the law is fundamentally unable to explain the entire emission spectrum of a black body – which is what it was attempting to do – as it contains both high and low frequencies. It’s simply wrong.
Also:
“Physicist Wilhelm Wien had shown that there was a mathematical relationship between the temperature of the black body and the frequency of the resulting emitted radiation. But it only worked for lower frequencies. ”
Wien’s law works for HIGH frequencies, but not low. The complete opposite of Rayleigh-Jeans law.
Good catch. Fixed!
Will to the rescue! Will is on it today. He’s worth every penny they pay him.
And using parabolas for trajectories of free falling objects is wrong. They are ellipses. Yet the flat Earth approximations work fine for normal human activities – even missile inercepts. They are considered the correct answer in advanced placement exams and beginning college physics.
You don’t appear to understand what you are talking about.
Sarah: You might explain your objection. All orbits are ellipses, even the segment of an orbit described by a thrown ball. If the mass of the Earth were concentrated at its center and there was no atmosphere, you could watch the ball fall all the way, go around the center, and return. The simple equations used in beginning physics classes are derived from an assumption of an infinite flat Earth. All those “Find the time in the air for a ball kicked over a goal at an angle of….” type questions are using parabolas to approximate ellipses. Things on parabolic trajectories do not return – they do not orbit an object.
The point is that there is nothing wrong with using approximations.
orbits are conic sections, the ellipse(+circles!), parabola, and hyperbola. All of them are legit orbits, gravity continues to govern their motion even if they never return!
but what does this have to do with the article?
I’m glad that you frequent this site. You are so knowledgeable about just everything that it’s just amazing. Dax for Hackaday editor!
>”Planck had originally turned his attention to the ultraviolet catastrophe in order to show that nature was not composed of atoms, but was continuous throughout.”
I don’t believe Planck was trying to disprove atoms either – after all, physicists had identified electrons by the time, and Planck was actually studying the oscillation of electrons as the fundamental oscillators or “vibrators” in the black body, as it was already understood that the source of electromagnetic waves/radiation was a moving charge, thanks to Maxwell.
It would make no sense to be denying the existence of atoms on the demand that nature should be continuous, while studying the behaviour of electrons. In fact, other sources claim that he turned his attention to the problem because he was commissioned to figure out ways to improve the efficiency of lightbulbs. (Helge Kragh, Max Planck: the reluctant revolutionary, Physics World. December 2000.)
What he was against was the probabilistic statistical interpretation of physics, not the idea of atoms.
It’s also a fact that Planck’s solution on the problem wasn’t actually accepted until Einstein came along. Before that, everyone thought that the Rayleigh-Jeans was the most reasonable explaination and fundamentally correct, and that there was simply some overlooked classical mechanism which would bridge the gap between it and the Wien’s-law.
To be more precise, what I understood of the story so far, Planck hated statistical mechanics because he didn’t like the probabilistic approach, but he had no choice but to use it becuse the Maxwell’s equations he was using didn’t explain how the vibrations could spread from one frequency to another, so that the energy could spread from one resonant mode or frequency to another and form the spectrum.
By Maxwell alone, due to the linearity of the equations, the outgoing radiation from a cavity should equal the incoming radiation both in quantity and in quality, but this was not the case. Using the classical equipartition theorem to let the energy spread resulted in the ultraviolet catastrophe result, so that was obviously not true either, and Planck didn’t believe in the equipartition theorem in the first place.
So he had to restort to “throwing dice”, essentially accepting that the energies get distributed randomly, and in order to make that produce the results they were observing, he had to quantize the energy of the oscillators so that the whole thing wouldn’t fall back to the equipartition theorem.
It wasn’t until later that quantum mechanics provided the non-linearities that would allow electromagnetic waves to self-interfere in such ways that they could exchange and spread energy across the spectrum.
“By Maxwell alone, due to the linearity of the equations, the outgoing radiation from a cavity should equal the incoming radiation both in quantity and in quality, but this was not the case. ”
Nobody -even back then- would expect Maxwell’s equations to be a complete description of electrodynamics, you still need some equation to describe the matter (say lorentz force for charged particles, or constitutive equations for current fields)….
to show a resistive material suffices to get (non-linear) quadratic effects: consider a core-less electromagnet and a corbino disc attached in parallel, and apply a sinusoidal voltage (or connect to an antenna and apply a single frequency wave…) and inspect the current through the corbino disc… I am sure Planck was aware of the concept of “heterodyning”, perhaps not by this engineering term but certainly by its mathematical meaning that multiplication in the time domain is convolution in the frequency domain (a basic result of fourier theory)…
https://en.wikipedia.org/wiki/Magnetoresistance#Geometrical_magnetoresistance
Also, the general formulation of the equipartition theorem still holds:
https://en.wikipedia.org/wiki/Equipartition_theorem#General_formulation_of_the_equipartition_theorem
Nice write up on this. Around what frequency does Rayleigh-Jean’s and Plank’s law diverge? Because it is easier to use mathematically Rayleigh-Jean’s law is a default go to. But I am not sure at what point is Plank’s law start taking over, less than visible light?
It’s around the ultraviolet range
No. Way less than visible light.
The Rayleigh-Jeans law only works in deep infrared towards radio frequencies.
https://upload.wikimedia.org/wikipedia/commons/thumb/1/19/Black_body.svg/303px-Black_body.svg.png
And of course it had to be a transparent PNG.
https://en.wikipedia.org/wiki/Ultraviolet_catastrophe#/media/File:Black_body.svg
The wavelength in that graph is micrometers. ¯\_(ツ)_/¯
“The wavelength in that graph is micrometers. ¯\_(ツ)_/¯”
What else should it be?
The Rayleigh-Jeans law actually starts to diverge significantly around 1 GHz (~30 cm). The whole point of it was the attempt to derive a radiation law from first principles using classical mechanical assumptions, and it proved that the classical assumptions were wrong or incomplete.
–>The Rayleigh-Jeans law actually starts to diverge significantly around 1 GHz.
The wiki that the graph is attached to says:
“Rayleigh-Jeans law accurately predicts experimental results at radiative frequencies below 10^5 GHz, but begins to diverge with empirical observations as these frequencies reach the ultraviolet region of the electromagnetic spectrum”
” at radiative frequencies below 10^5 GHz”
“begins to diverge with empirical observations as these frequencies reach the ultraviolet region”
10^5 GHz is 2.9 µm which is deep infrared. The Rayleigh-Jeans law is predicting the spectrum all wrong well before you even get to the visible range, and it goes ridiculously wrong in the ultraviolet – which is why it was called a catastrophe.
“Accurate” seems to be loosely interpreted by the article to mean “within an order of magnitude”.
Well, short infrared. Apparently 3 µm is still considered SW-IR
Here’s a comparison of the three laws:
https://upload.wikimedia.org/wikipedia/commons/thumb/7/72/RWP-comparison.svg/750px-RWP-comparison.svg.png
If you are going to play with Planck and Boltzmann you better get the terms right :-) “hottest temperatures” should be :highest temperatures. No such thing as a hot temperature. That’s like saying “Man, distances are long today!”.
Fine. Fixed!
Thank you for writing this article. I heard a very abbreviated version of it in a college class about 20 years ago and am glad to both hear it again and to get some further details. :)
(And wow!!!! So many nitpicks . . . )
“(And wow!!!! So many nitpicks . . . )”
This is an article that takes on an interesting chapter in the history of physics, then largely glosses it over with a handwave and talks about everything else, and where it does touch the topic it makes several factual errors and serious omissions.
If the topic had been something like “Bolztmann vs. Planck, the constant argument”, there would have been less complaints.
There were no factual errors. Just typos.
I call mistyped letters typos, you call misleading sentences typos.
And I still think the assertion that Planck was trying to show the world isn’t made of atoms to be incorrect.
The term ‘misleading’ is subjective. Facts are objective. For the atoms issue, in his early years, Planck is on record as saying that the atomists point of view was a “dangerous enemy of progress” and would “have to be abandoned in favor of the assumption of continuous matter”.
Maybe I could have worded it a little better…if I were writing the article again I would pay more attention to the atom area. But as long as the reader understands that Planck was drawn to cavity radiation because it offered a path to undermine the atomists’ statistical-mechanical description of matter, they will have an accurate understanding.
I agree with Dax. On main stream media you can get away with wishy washy stuff, but when you are reporting technical areas, that is kind of writing is in the way.
It would be helpful to ideally have somebody else (an editor?) proofread it before posting an article. It’s an article that’s mostly targeting a technical audience about a technical topic. Nothing personal against the author, just trying to help fix glaring issues at first read that should be resolved by the author or editorial staff. Not sure how else to conveniently comment on the article except, well, commenting on the article.
You want Hackaday to turn into a technical journal?
A subscription to Nature costs $200 a year. IEEE membership, which includes a subscription to IEEE journals, is $480 a year. Hackaday commentors get offended when I suggest you whitelist hackaday in your ad blocker.
The commentors in this article have a delusion that this is a technical article about a technical subject directed towards a technical audience. This article does not pretend to be that. It is a general science article directed at the general population. There are no technical errors, and apart from the commentors, no confusion between colloquial speech and technical speech. The amount of hate this article has received – and Hackaday articles in general – is horrifying, reinforcing my belief that hackaday commentors are only experts in self-proctology.
” This article does not pretend to be that”
Then why is it called “The Ultraviolet Catastrophe” when it actually tells next to nothing about it?
“There are no technical errors”
Except the ones pointed out and fixed.
Thank you for more eloquently saying what I was thinking.
Btw, what’s your problem Brian? You treat the whole issue like some of those clueless restaurant owners on Kitchen Nightmare, where they make shitty gruel for food in a dump of a bistro with an attitude problem and then complain that the customers don’t leave tip.
Sure, you’re not a four michelin star restaurant and you’re not even trying to be, but have you no self-respect and work ethics? Is this it? Just post some half-assed filler articles by people who are as cheap as they are incompetent, and then complain that readers don’t turn off their adblock?
You’re running a site whose main content is provided by other people anyways, free for you. As for the whole ad business in general, you’ve got a nice unethical setup going on: in order to check if the article is worth paying for, I have to read the article, which means you get ad-revenue, which means I’ve rewarded you regardless of the quality of the article. Does that encourage you to publish good articles; no it doesn’t.
If you seriously want to make money ouf ot me, ask me. If you got something worth paying for, I will. The thing is, you don’t want to provide because it costs you more, so you’re taking the easy way out and trying to exploit me otherwise. You got nothing to complain about.
Somebody got told by the new boss to target a larger audience..
That’s a bit of an unfair comparison though. Nature and IEEE do not advertise, nor do they serve as for profit endeavors. They are fundamentally distinct and they and Hackaday serve different audiences and this site exists as a commercially oriented, “pop culture” yet somewhat specialized blog / advertising vehicle. This was a nice article but there were a number of basic errors that detracted from the experience of reading it as well as diminishes the perceived quality of the “paid by the article” author.
I agree with Dax, that there were multiple basic errors here that should have been caught by proofreading the article prior to publication. I am not directing hate towards the article or the author when I or others point that out. It’s not personal, most of the readers of your site are technical in nature and when you combine that with the lack of person to person interaction as well as the lack of tone that is muted by turning the communication into words, it is easier to perceive malice and negativity when there was not (intentionally) any present.
I cannot speak to Dax’s analysis of the rest of the article in terms of his concerns with it as this field is generally outside of my specific in depth knowledge.
And the editors of the site have a delusion that they’re writing for the audience of the Daily Mail or Buzzfeed. They’re not.
Wow, quite the attack, Brian~ why all the anger? I’m a regular commenter, and have read every article ever published on your site and, although the lack of proof-reading frustrates me at times, I’ve overlooked it for years, because the content you deliver is worth a little frustration. There are still errors in the article (grammar), but again, they can be overlooked. What’s hard to overlook is Mr. Brian Benchoff, whom I respect deeply, aggressively defending an error-filled exposition rather than agree that proof-reading would be helpful, then to top it all off, you insult ALL hackaday commenters. I wasn’t going to comment, until you insulted me personally, as well as any reader who’s ever commented on this site. (P.S. see: http://grammarist.com/usage/commentator-commenter/). Much respect and appreciation to Brian Benchoff and Hackaday, but a little less today than yesterday.
There’s a gif for that.
The biggest error is the ridiculous abuse of the nature of a black body cavity, especially by the commenters.
Eewww that is disgusting!
But how did you even gain access to his butt cheeks?
XD
As a student of physics myself, I found this article very fun to read – It took me back to my undergrad classes. I feel it is well written to reach a wide range of readers, while leaving some of the more technical information out that only a few of us would really care about or appreciate. Good article, keep it up!
The best part about this article are the comments. Thanks for refreshing our memories of the Planck÷Boltzmann battle. Really interested to know how Planck got the final equation in his strenuous weeks.
Dax for president. He must be huge.????
Google is huge, not me.
LibGen is even bigger. ;-)
I assume Rayleigh scattering fits in here somewhere?
This article reminds me why I changed my major from Science to EE and then CS. You lost me right around the point where you mentioned the word “entropy” for the first time :)
Thank you for the interesting article. I’m looking forward to the next one.
I enjoyed the article, cept not listing actual formula: wavelength (lambda) = orbital volt difference / Planck’s constant. (Learned from “Life and Times of Einstein”)
A very well written article, if one is interested in knowing historical aspects of the development of certain scientific issues. Planck, as also a section of scientific luminaries of that time, was against the idea of atoms.
The article clearly conveys what it intended to do. Some simple errors were readily fixed when pointed out by commentators.