It is funny how sometimes things you think are bad turn out to be good in retrospect. Like many of us, when I was a kid, I was fascinated by science of all kinds. As I got older, I focused a bit more, but that would come later. Living in a small town, there weren’t many recent science and technology books, so you tended to read through the same ones over and over. One day, my library got a copy of the relatively recent book “The Amateur Scientist,” which was a collection of [C. L. Stong’s] Scientific American columns of the same name. [Stong] was an electrical engineer with wide interests, and those columns were amazing. The book only had a snapshot of projects, but they were awesome. The magazine, of course, had even more projects, most of which were outside my budget and even more of them outside my skill set at the time.
If you clicked on the links, you probably went down a very deep rabbit hole, so… welcome back. The book was published in 1960, but the projects were mostly from the 1950s. The 57 projects ranged from building a telescope — the original topic of the column before [Stong] took it over — to using a bathtub to study aerodynamics of model airplanes.
X-Rays
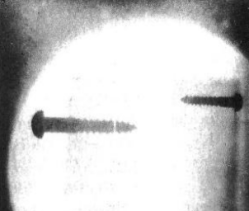
You needed a few items. An Oudin coil, sort of like a Tesla coil in an autotransformer configuration, generated the necessary high voltage. In fact, it was the Ouidn coil that started the whole thing. [Harry] was using it to power a UV light to test minerals for flourescence. Out of idle curiosity, he replaced the UV bulb with an 01 radio tube. These old tubes had a magnesium coating — a getter — that absorbs stray gas left inside the tube.
The tube glowed in [Harry’s] hand and it reminded him of how an old gas-filled X-ray tube looked. He grabbed some film and was able to image screws embedded in a block of wood.
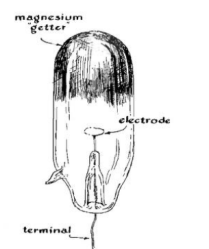
However, 01 tubes were hard to get even then. So [Harry], being what we would now call a hacker, took the obvious step of having a local glass blower create custom tubes to his specifications.
Given that I lived where the library barely had any books published after 1959, it is no surprise that I had no access to 01 tubes or glass blowers. It wasn’t clear, either, if he was evacuating the tubs or if the glass blower was doing it for him, but the tube was down to 0.0001 millimeters of mercury.
Why did this interest me as a kid? I don’t know. For that matter, why does it interest me now? I’d build one today if I had the time. We have seen more than one homemade X-ray tube projects, so it is doable. But today I am probably able to safely operate high voltages, high vaccums, and shield myself from the X-rays. Probably. Then again, maybe I still shouldn’t build this. But at age 10, I definitely would have done something bad to myself or my parent’s house, if not both.
Then It Gets Worse
The other project I just couldn’t stop reading about was a “homemade atom smasher” developed by [F. B. Lee]. I don’t know about “atom smasher,” but it was a linear particle accelerators, so I guess that’s an accurate description.
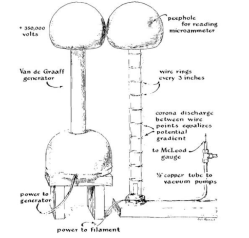
I doubt I have the chops to pull this off today, much less back then. Old refigerator compressors were run backwards to pull a rough vaccuum. A homemade mercury diffusion pump got you the rest of the way there. I would work with some of this stuff later in life with scanning electron microscopes and similar instruments, but I was buying them, not cobbling them together from light bulbs, refigerators, and home-made blown glass!
You needed a good way to measure low pressure, too, so you needed to build a McLeod gauge full of mercury. The accelerator itself is three foot long, borosilicate glass tube, two inches in diameter. At the top is a metal globe with a peephole in it to allow you to see a neon bulb to judge the current in the electron beam. At the bottom is a filament.
The globe at the top matches one on top of a Van de Graf generator that creates about 500,000 volts at a relatively low current. The particle accelerator is decidedly linear but, of course, all the cool particle accelerators these days form a loop.
[Andres Seltzman] built something similar, although not quite the same, some years back and you can watch it work in the video below:
What could go wrong? High vacuum, mercury, high voltage, an electron beam and plenty of unintentional X-rays. [Lee] mentions the danger of “water hammers” in the mercury tubes. In addition, [Stong] apparently felt nervous enough to get a second opinion from [James Bly] who worked for a company called High Voltage Engineering. He said, in part:
…we are somewhat concerned over the hazards involved. We agree wholeheartedly with his comments concerning the hazards of glass breakage and the use of mercury. We feel strongly, however, that there is inadequate discussion of the potential hazards due to X-rays and electrons. Even though the experimenter restricts himself to targets of low atomic number, there will inevitably be some generation of high-energy X-rays when using electrons of 200 to .300 kilovolt energy. If currents as high as 20 microamperes are achieved, we are sure that the resultant hazard is far from negligible. In addition, there will be substantial quantities of scattered electrons, some of which will inevitably pass through the observation peephole.
I Survived
Clearly, I didn’t build either of these, because I’m still here today. I did manage to make an arc furnace from a long-forgotten book. Curtain rods held carbon rods from some D-cells. The rods were in a flower pot packed with sand. An old power cord hooked to the curtain rods, although one conductor went through a jar of salt water, making a resistor so you didn’t blow the fuses.
Somehow, I survived without dying from fumes, blinding myself, or burning myself, but my parent’s house had a burn mark on the floor for many years after that experiement.
If you want to build an arc furnace, we’d start with a more modern concept. If you want a safer old book to read, try the one by [Edmund Berkeley], the developer of the Geniac.
















No mention of the Anarchist’s Cookbook?
I’ve got an old, old, mimeographed copy of the Cookbook. Supposedly before the three letter agencies started tinkering with it. I need to get it out and compare it to the old BBS ones and what’s floating around now. And no, never made anything fun from it.
If you believe someone conspired to put misinformation into the anarchists cookbook I suggest Occam.
The Hippy was really really stoned.
He printed stuff he heard from other stoned people.
Didn’t actually do all the things he taught, not even once.
People still have old hard copies.
It hasn’t changed.
It’s just dangerously wrong and always has been.
If you want to look deeper for conspiracies behind AC:
Timothy Leary was a CIA asset! Fact. A generation of rebels set their brains alight.
Is good fun! Just not all the time.
People with a few IQ points to spare? Happier dumber? Beware happy. Too much is too much. The dumb feeling the next day? It never goes away, you just get used to being a dumber you.
I’m not going to say which part, in particular, of that book is known to kill assholes.
It’s one of those: ‘He tried to do what? Died? Good.’ things. F-em.
The projects in it are less interesting than the one’s mentioned here.
Maybe ease off the banadine?
Reminds me of when I built a Jacob’s ladder on the back of my bedroom door using a 10,000 volt transformer from a neon sign. Or when I tried to distill vodka using an open flame to try to make pure alcohol and my home made condenser leaked exploded and started a fire in garage and sent a shard of glass into to my finger. The deep cut left a scar that was clearly visible for 30 years and faintly visible after 60 years . Luck is real
I did the 1L boiling flask / copper condenser thing too. Second batch had similar result as yours, wheat paste seal at cork failed!
But if i had to pick a book it would probably be ‘fireworks principles and practice” when i was 11. The one that got me in the most trouble was the ad in back of a magazine “send a dollar and get a pamphlet with 10 recipies for tear gas”. Turns out i shoulda done that one outside.. duh
The trick with still condensers is that they need to be free-flowing — you can’t hook it up to the output vessel with a tight seal. In your 12-yr-old brain, it might seem like all of that steam just shrinks back down to liquid, but there’s excess steam, for sure.
If you do make it airtight, you get overpressure in the system, and something made of glass explodes. Fortunately, it was the collection jug, and nobody got hurt. But my parents had to repaint the kitchen ceiling when they sold the house because of the water stains.
(I think they left it until then out of superstition or some kind of strange pride as much as laziness.)
Boiling water drives trains, folks.
I’ve commented enough, but I absolutely MUST get one in regarding something you just said–“10,000 volt transformer…neon sign”.
[Long ago, I bought two neon sign transformers, to make a ‘prank’ Jacob’s Ladder out of a TV “rabbit ears” antenna, to ‘liven-up’ TV-watching parties. Then I read the boxes…mine are rated at 12 000 V, 20 ma.]
These things rank right up there with the deadliest devices or compounds you can own or work with.
Do the math: 12 KV X 20 ma = 240 WATTS ! !
(For anyone who might be mathematically challenged and / or have a slight problem with the units, that equates to almost ¼ kilowatt which would possibly pass through (some)one’s heart)
This is way more than enough to ruin anyone’s day–who happens to be unfortunate enough to get their heart between the two leads of that neon-sign transformer.
Mine are still brand new, still in original boxes–all 12 000 volts and 20 ma. worth of them.
I won’t consider using them (death, particularly my own, scares he Hell out of me), and I have some very strong social-responsibility-type feelings about even selling them to someone else; even though I’m absolutely certain that there are more than enough Darwin-Award-idiot candidates out there who would pay top dollar for them.
I got two with 15kV each. …and actually pulled it through.
Just had to overcome the fuse, it didn’t both of the turned on simulatenously.
Arc was wonderful. I had 10mm stainless steel rods.
Then I put some floral wire to the top to “shape” the arc.
It burned away like sparklers within seconds.
My carpet reminded me many years, that these things can melt iron wire within seconds :D
Bought a copy in ’75. Tried a recipe in ’78, and got kicked out of a country. YMMV.
Actually built the XRay machine… But with an old radio tube and Model A ignition coil. Also turned the arc generator into an thermal plasma rocket engine. But, dollars for vacuum pumps were beyond eighth grade allowance. Great article. Thanks for the trip down memory lane!
I had a high school friend who built one. I never visited him while he used it. Not because I knew better … just luck …again. I don’t know if he suffered any ill effects
I checked that book out of the school library over and over. The all metal zinc and sulfur powered rocket. A home made electrocution machine, er, electrophoresis machine. A very cool analog star tracker, and the Vortex Tube!
It was the best. It didn’t imply you could make these and other things, it said with certainty that you can do it. It had REASONABLE safety precautions for reasonable people. Not modern education legal protection precautions, like a blast shield if you are poking a balloon, or the micro-chemistry that has replaced the beakers, flasks, and Bunsen burners in the high schools of the USA.
I have a little bin with small photo-multipliers and motors and other things to some day make the star tracker!
What were your other favorite projects from the book?
The star tracker should be a lot easier to do nowadays; precision metrology has become cheap. But life is short!
In the book, what I call the star tracker is a pair of prisms on voice coils (IIRC) to cancel “twinkling” or the small apparent motions due to the air. There was something about the photo-tube setup that never let go of me and I have sketched up various spinning masks or masked plane mirrors and high gain photo-multipliers ever since.
On big telescopes of the day, the astronomer sat in a seat near the focus and the photographic place was in a big 2-axis holder with x and y dials. They looked through an eyepiece a bit off-axis and used the dials to keep a guide star centered since the full telescope could not respond to small corrections happening quickly. But in a modern amateur telescope the drive can handle it.
The photo-multiplier tube doesn’t need any integration time and the optical scheme with spinning parts can be producing X and Y error voltages at whatever the spin rate and with pointing a fraction of the apparent diameter of the tracking star. I like the idea of no computer. Find a star and hit GO and you get a negative feedback lock.
Oddly , I did use an old TV tube. The diode tube ,as it had the least metal to get in the way, to produce Xrays when I young . The tube did glow. The exposed film (contact Paper) produced a very poor picture. I was a little older than you then. I was all of twelve. I have lived to tell the tale. I even had normal children.
Careful, you’ll anger the safetyists
You have had an independent third party verify the normality of your children?
I’m skeptical.
You are here.
They have both had children of there own. One is a speech pathologist , The other teaches at a university . I suppose that is not necessarily considered normal.
I’ve got a similar volume called “The Boy Electrician” which as well as X-Raying your pet animals explains how to build and connect various dangerous contraptions directly to the mains. Childhood these days is such a sanitised affair
Even learn some Darwin. Kids these days have it easy.
Pets today are so much safer.
I have fond memories of the column, and this book, as well as the various “Boys Book of …” . Kids can’t have any fun these days. Mercury was a very cool thing to play with :-) I even had two chemistry sets with chemicals that now would cause a parent to be screaming for a lawyer. I had such fun as a kid, learning about all of these “dangerous” things, and I still have all my parts intact.
Survivorship Bias? :)
Science hobbies were never a major cause of childhood mortality. People are just superstitious about mercury and similar risks.
Might get a book out of it.
https://en.wikipedia.org/wiki/Erethism
It’s pretty unlikely. As with asbestosis and silicosis, it’s generally people who are exposed to mercury day in and day out for decades who get erethism; historically it’s an illness of hatters and dentists, not physicists and meteorologists. Liquid mercury poses almost no risk and was conventionally taken by mouth as a laxative in the 19th century, similar to mineral oil. Mercury vapor is worse, but still not in the same league as the hatters’ mercury salts, which are not in the same league as organometallic mercury.
Mercury plus a hot surface is fun. Watch it jump around as it boils away. Neeto! (don’t do this)
I had more access to mercury as a pre-teen than was safe.
But I knew not to put the soldering iron into mercury.
Dad was a pyro too. Knew the spots a boy could find excess danger.
Steered me clear of nitroglycerin, right to the reloading aisle at the gun store.
Made the right choice. Honesty.
Didn’t say ‘go to gun store’, said ‘nitrocellulose much safer’. Knew I would get from B to A.
With deniability to mom. Not that I’d ever have ratted.
He hadn’t said anything ‘in so many words’ anyhow.
Mom had sworn him to silence regarding his own pyro exploits w me in particular.
I digress.
But I also knew many kids that would have taken the soldering gun to mercury _right_after_ being told not to.
Big magnifying glass on mercury thermometer bulb kind of super genius’.
“Steered me clear of nitroglycerin, right to the reloading aisle at the gun store.” consider that smokeless powders are about half nitroglycerin absorbed by the nitrocellulose :-)
I don’t think that’s how it works.
IIRC nitrocellulose is made the same way as nitroglycerin, not with nitroglycerin.
It’s the fuming acids that were impossible for middle school me to get.
Bloody fascists!
Second amendment!
Freedom!
All the double base powders are around 50% nitroglycerin by weight. https://www.britannica.com/technology/double-base-gunpowder
Yeah…played with mercury too 😂
In the early 80’s, a high school physics book had a simple illustration of a hot-plate boiling mercury out of a jar, and that the mercury vapor blocks x-rays… invisible to the eye, but mercury vapor would be there much like steam from water. My inquisitive friend and I had lots of mercury (from old thermostats, and furnace limit switches), so we put a couple ounces into a jar and heated it. Yes, Hg does boil easily and rather vigorously!
Stong’s article collection was later republished on a decently-organized CD.-ROM
Still listed on Amazon: https://www.amazon.com/Scientific-Americans-Amateur-Scientist-Science/dp/0970347626
But I’m sure it’s also available online. But many (most?) complete issues of Scientific American are available online anyway, through the legit source. Some fascinating reading. Not at all like the current fluff.
And I stand corrected: it’s not Stong’s articles, it’s the later Carlson ones.
The book I read as a kid, the old “Handbook for young inventors and experimenters” had a chapter about shaving a graphite pencil bare and connecting the ends to an extension cord. This was fixed onto a piece of wood with metal stands fashioned out of a tin can, and the cord was then plugged into a wall socket.
What it didn’t mention was, back in 1940-something, it was still common for households to have low voltage sockets with 24 volts coming from a battery or a transformer, for operating reading lamps, and small appliances like electric shavers in the bathroom. I think it did mention it was a “radio socket” or some such, but it wouldn’t specify what that was, so a modern reader could easily mistake it for a regular wall socket. If you repeated the experiment today, the pencil lead would probably explode instantly.
I think a pencil lead (the traditional kind with graphite and clay, not a polymer-based fancy-shmancy one) can probably handle over 100 watts. My multimeter claims that this pencil I have here on the desk (an HB) is 4kΩ, and that a short piece I broke off the end (about a fourth of it) is about 1.9kΩ. This suggests a contact resistance of about 1.2kΩ and a pencil resistance of about 2.8kΩ. If you put 240Vac rms across 2.8kΩ you’ll get about 90mA rms and 20 watts, so not only won’t the pencil lead explode, it will probably endure indefinitely. On 120Vac it’ll be more like 5 watts, probably not even enough to glow.
Maybe if you use a 2B or 4B pencil, the results would be more exciting.
Graphite has the curious property of having a negative temperature coefficient of resistivity, and I think the clay also starts to conduct electricity when it gets hot enough (how Nerst lamps work), so you get this runaway effect going.
But what probably happens is that the lead will start to glow white hot and then suddenly burst apart from the thermal expansion stresses.
These are good thoughts.
It’s true that graphite has lower resistance when it heats up (because it’s a semiconductor) but carbon-composition resistors are basically painted pencil leads, and “second breakdown” isn’t a significant problem with those, so I don’t think the positive feedback effect is very strong. Remember that Stefan–Boltzmann thermal radiance goes as the fourth power of temperature, which works as a strong negative feedback on heating.
I doubt the lead will get to white-hot before it melts; the graphite is bound together with clay, and cheap clays start to sag at only orange heat, though a good ball clay or kaolin can indeed reach white heat. I don’t see why pencil lead manufacturers would spring for the good stuff, though.
It’s true that any material becomes a conductor once you get it hot enough, but Nernst lamps are special in that zirconia can do it without melting; the oxygen ions can migrate through the crystalline matrix while the zirconium ions remain in place. I think Nernst would have used a cheaper oxide for his globars if he could. (As I understand it, modern globars are usually zirconia or carborundum.) So I don’t think the clay in the pencil lead will start conducting, even if it’s white-hot.
Heat loss due to conduction to air will be dominant until very high temperatures. The Stefan–Boltzmann constant is so small that the effect only really starts to pick up above 3000 Kelvins and that’s already too hot, especially for carbon in air.
Old timey Edison carbon bulbs were filled with inert gas to actually cool down the filament and prevent it from burning up so easily. They were operating at around 2300 Kelvins.
Also remember that with a thick pencil lead, the heat has to first get out and reach the surface before it can radiate, so the interior of the lead can become significantly hot even as the surface remains relatively cool. This creates tremendous thermal stress within the material, and I suspect this might cause the pencil lead to explode when it’s suddenly heated with kilowatts of power.
The negative temperature coefficient is also the reason why carbon filament lamps were so dim. The heat loss from the filament increases somewhat linearly with temperature, while the amount of power the filament will produce goes up exponentially.
If you plot for example y=x versus y=x^2 to represent the heat loss and the filament power, you see that the linear function starts out greater, but at some point the second order function overtakes it. Suppose we apply a certain voltage to the filament – this is equivalent to adding a constant offset, like so:
y1 = x
y2 = x^2 + 0.16
We see that the functions cross at two points: 0.2 and 0.8. Between those points y2 will be lower than y1, so the filament can lose more heat than it increases in power. That means the filament temperature cannot rise above 0.2. If for some reason the filament got hotter than 0.8, it would start to heat up faster than it can shed heat, and the temperature would run away.
If we keep increasing the applied voltage, the two points will come closer together. If we raise the “voltage” to 0.25 then the two functions cross at a single point at 0.5 and there is no stable temperature where it could settle – the filament burns almost instantly. Metal filaments don’t do this – they’re stable up to the melting point of the metal because they have a positive coefficient of resistivity.
For the design of the bulb then, you had to run the carbon filament at a low enough temperature that an increase in the grid voltage would not send the bulb over to become unstable. The voltage of an Edison DC circuit would vary greatly depending on local load, so if the margin was too tight, all your bulbs might have gone pop in a cascade if one light went.
You could add the Stefan–Boltzmann law into this, but as noted above, it would not have significant effect until past the point where the filament gets destroyed.
https://chemistry.stackexchange.com/questions/181377/how-does-graphite-content-affect-the-conductivity-of-pencil-lead
If we assume the lead is 15 cm long and 2 mm wide, I get about 20 Ohms of resistance by calculation. Someone else on google did the experiment and got about 15 Ohms, so it seems to be in the right ballpark. It should yield about 2-3 kW of power, plus the fact that the resistance halves when it reaches 1000 C, so you’re more than likely to trip the fuses before the pencil explodes.
Though on a 120 Volt circuit it might work. You get about 1500 Watts so the circuit should take it – and the pencil not.
Hmm, now I’m tempted to try the experiment myself. 2kW wouldn’t be enough to trip my breaker but 3kW would. It would be plenty to melt the clay, though.
Make sure you take a vid.
Just in case you die hilariously.
The world will get to laugh.
That’s 2-3 kW when the graphite is cold. You could get up to 6 kW if all goes “well”.
Best start with a thinner piece and lower voltages.
It’s easy to play with this with a 0.5 mm mechanical pencil lead and an adjustable bench supply: It draws a couple of amps at around 10-15 volts depending on grade of lead (2H, HB, etc).
It’s easy to make it a stable dull red, but if it goes into the yellow it starts thinning rapidly in the middle — a runaway process that causes a bright center section for a few seconds before it completely burns up.
Pencil leads make better arc lamp electrodes. Put a conventional 100 watt tungsten lamp in series with a pair, power with 120Vac, and draw the arc. Works great, doesn’t erode the lead very fast. I’m sure it throws more UV than is healthy for corneas, but that’s what myopia is good for (always-on UV protection).
>It’s easy to make it a stable dull red
That was the point of the experiment in the book – when hooked up to the proper supply it would simply smoke a bit and then turn red, demonstrating the principle of electrical heating in a relatively safe manner.
The book also had other interesting instructions, such as throwing old zinc-carbon batteries in the fireplace to emit zinc vapor into the smoke stack, to clear off soot buildup by catalytic action. It didn’t mention though that zinc vapor is poisonous – and the fact that modern disposable batteries have metal lids instead of wax paper, so they will explode with pressure.
Climbing trees is dangerous. So is swimming in the ocean. Even standing out in the sun. Why should a little danger put us off from doing such interesting experiments?
“but my parent’s house had a burn mark on the floor for many years after that experiement”
I just assumed that was normal behaviour, right?
Mr. Wizard taught me how to build a fire extinguisher out of vinegar and baking soda. That’s when we got cable TV. What he failed to mention was that the vinegar would dissolve the glue in the wafer board floor in the basement causing my dad to not be happy when he stepped through it. If you’re going to build a fire extinguisher you need a reason to use it. To this day he’s not aware why we had to replace the dirt and OSB with concrete.
Future engineers and hard scientists have a ‘pyromaniac’ and ‘explosive pyromaniac’ psychosocial development stage.
Perfectly normal. Only a problem when it extends into adult court.
That explains a lot….
My dangerous book was the 1940’s “Chemistry & You” that told you how to build various explosives. Of course, we tried several in the name of science.
My dangerous book as a kid was “Rocket Manual for Amateurs” by Bertrand Brinley (Ballentine Books, 1960). The things I learned to do from that book back then, if done today, would bring SWAT teams, FBI helicopters, and K-9-driven armored tanks swarming all over me and my chemistry lab in my parents’ basement.
Beetrand Brinley was also the author of the Mad Scientist Club books.
I remember those books.
The worst influence for me was the Britannica.
That encyclopedia had modern and historical formula for many explosives.
Just running around ‘playing war’ with an expended LAW rocket tube (bazooka) would likely be ‘frowned opon’ these days.
To say nothing of lining up on the cop car going by.
To be fair, we were about 6-8.
I have a copy of that, it also has a model rocket that’s basically just a spear with zinc/sulphur propellant, and a nuclear magnetic resonance spectrometer.
My dad built and launched dozens of those zinc/sulfur rockets. The body was a piece of copper plumbing tubing with the nosecone formed by crimping, soldering, and filing. No parachute or recovery system at all. You just light it and FWOOOOM off it goes to plunge like a dart into some neighbor’s yard or roof. And even better, Zn/S will sometimes spontaneously ignite, and if it does so at the top of the rocket rather than the exhaust, it’ll explode and shoot copper fragments through anyone nearby.
Estes Rockets was a revolution in safe rocketry.
I remember the old Estes catalogs with their horror stories about the hideous dangers of zinc-sulfur rocket engines…
For those seeking more of this era of scientific construction, please see the 1938 book _Procedures in Experimental Physics_ [https://archive.org/details/ProceduresInExperimentalPhysics/page/n1/mode/2up]. Both this book and Stong’s column were illustrated by Roger Hayward. The book is from before the (post-WW2) era of the commercialization of scientific apparatus. At the time if you wanted experimental gear there was no choice but to make it yourself.
The most interesting lesson I learned from the book it that the plastic material of the era was glass. It had a position related to that of 3D printing today. If you needed an arbitrary shape, you made it from glass. Accordingly chapter 1 is on glass blowing and chapter 2 is on lens grinding. Chapter 3 is on vacuum systems. (Hint: these day refrigeration service pumps make for readily available roughing pumps.) And chapter 4 is on surface coating, including sputtering and evaporation. So if your gold-plating SEM sample prep device could be shop-built in the 1930’s, there no real excuse to consider it out of bounds today.
YES. THIS. I actually found a pulled-from-circulation ex-library copy of this on ebay and snatched it up some years back. It is everthing I remembered it to be…information you simply can’t fund anywhere else, anymore. That book, the Amateur Scientist book, and anything by Alfred P Morgan, Rufus P Turner, or Forest Mims had profound influence on me.
I loved that book.
I have a “The Boy Electrician” book from the 30s, with a chapter on things to do with an x-ray machine. It does warn you that you are probably overdoing it if your skin reddens.
And then there was “An Unusual kind of gas laser that puts out pulses in the ultraviolet, Scientific American, Amateur Scientist Column, June 1974, A simple nitrogen laser easily constructed by an amateur”
Man, I thought the 1 board, 2 nails, and a power cord hot dog cooker was dangerous.
It was, and still is (dangerous, that is).
The fact that you had enough common sense to think so is why you’re here to write your comment.
The ones without your level of common sense are the posthumous winners of a Darwin Award.
Board and nails?
Unnecessary complications.
Are you some sort of amateur OSHA inspector?
Tin the ends of wire with silver plumbing solder. 3/4 to 1 inch. Hook shape for Bratwurst or similar.
Using forks instead of nails is much better.
These things have also been produced and sold commercially for quite a while. But those versions (at least the later ones) have a lid with an interlock that takes off the power when the lid is opened.
There was an older edition of “700 Science Experiments for Everyone” that had fun stuff like the ammonium dichromate volcano (with magnesium added!!), carbon arc with battery carbons (cord was wrapped around the carbon rod and fastened with electrical tape so you could hold them in your hands!!), etc. Had some less hazardous activities too, like making a scale sensitive to a few milligrams, alcohol lamp, turning old lightbulbs into flasks…
Yep, that’s the one with the carbon-arc “furnace”. Compiled by UNESCO. Experiments for countries where equipment is expensive and life is cheap. (“Build a handy rheostat for line current by wrapping wire around two rocks in a pan of salt water! Make sure the pan isn’t metal.”)
From the article–
…”An amateur named [Harry Simmons] had described his setup complaining that in 23 years he’d never met anyone else who had X-rays as a hobby. Oddly, in those days, it wasn’t a problem that the magazine published his home address…”
Oh, for the good old days when paranoia did not reign supreme–because it has been forced on us…
I’ll never forget the evening my home telephone rang, and I found myself on a conference call with four or five gentlemen who identified themselves as engineers with NASA-Ames.
It seems as though they were so taken with a full-detail (including a custom-designed, magazine-cut-out meter face) article I had written for Radio-Electronics magazine** that they had decided to build several units…but before they did, they had one small problem with implementing my design, and wanted my help.
What was the engineering reason, they wanted to know, that I had decided upon the use of 2N2924 transistors for the monostable multivibrator which was at the heart of the design?
After letting them know how flattered I was that they considered my design worthy of duplication, as gently as I could I informed them that there was a very elegant reason I had decided to use 2N2924s: I had lots of them in my ‘junk-box”.
They agreed with my decision that it really didn’t matter as long as very good basic transistor parameters were met (β, etc.). We had laughs all around; I made several good friends; and priceless memories.
Here’s the anti-climax–
as our conversation was winding down, it occurred to me to ask how they got my telephone number. “Oh, that was no problem”, one said. “We simply called Radio-Electronics and they gave us your phone number and your address”.
We will, sadly, never get those days back again.
————————————————————————-
**“Build: Low-Cost Solid-State Tach-Dwell-Voltmeter”, Radio-Electronics, Vol. XXXIX, No. 6, June 1968
(A subsequent, updated, article was written, using an MC789P hex inverter instead of discrete transistors–
“Dynamic Dwell/Tachometer”, Electronics World, Vol. 85, No. 5, May 1971)
What a fantastic story. Thank you for sharing that.
I’m also surprised I’m alive today.
I’m proof that this is an unlikely universe considering how many things I did between the ages of 12 and 18 that definitely would have killed me had anything gone wrong.
I’m glad I never seriously hurt myself or anyone else. Living near an abandoned quarry is a double edged sword of opportunity and separation from bystanders.
I do still have the letter with the fine I got for violating controlled airspace when I was 13 though. In a time before national terrorist attacks, the FAA could afford to have a little chuckle when an object with some kid’s address floated out of the sky onto an airfield…
The book that almost killed ME was some variation on “Chemical Magic”, published in the 1950s. Stupid flare-powder recipes using sulfur and chlorate instead of perchlorate.
I never could get the ingredients do the “ghostly glowing writing” experiment where you dissolved white phosphorus in carbon disulfide, added a little olive oil, and painted it onto paper. (Fortunately, in retrospect.)
This reminds me of David Hahn, “the Radioactive Boy Scout” (R.I.P.)
His book was The Golden Book of Chemistry Experiments and he was inspired to collect enough radioactive material to turn his shed into a superfund waste site.
Does death by parents count for “this book killed me”?
Original Harper’s article “The Radioactive Boy Scout”. https://harpers.org/archive/1998/11/the-radioactive-boy-scout/
David Hahn
https://en.wikipedia.org/wiki/David_Hahn
The Golden Book of Chemistry Experiments
https://en.wikipedia.org/wiki/The_Golden_Book_of_Chemistry_Experiments
As a kid, there comes a time, when you realize you are in over your head and what follows is likely to be life changing.
You folks obviously missed the 2-volume set, “The Chemistry of Powder and Explosives,” by Tenney L. Davis. They are classics in the world of pyrotechnics. A bible of information about fireworks. Armed with Davis’ wisdom, and a supply of chemicals, my brother and I put on displays for a local beach club. In those days chemical companies had no problems selling us 25-pound bags of potassium nitrate and chlorate, as well as magnesium and zinc dust. Those were the good times. For a while we sold pyrotechnic fuse in 50-foot rolls and white-smoke powder by the pound. We also built a cannon and put a piece of wooden closet rod through the sides of our grandfather’s barn. Good times!
When I was young, I decided to build an oxy-hydrogen torch with the gases provided by hydrolysis. I put together an old model train transformer, a Mason jar, a rubber cork designed for a flask, and a jet tube that I made myself from glass tubing. I knew the gas would take a while to form; so I left a propane torch burning such that it would light the gas on fire as it started coming out of the tube, and went upstairs to watch some TV.
Then I went back down to the basement. Nothing was happening yet, so I put my head close to the contraption to listen for the sound of the bubbles forming. At that moment the gas ignited…
Stupidly – I knew better but wasn’t thinking – I hadn’t separated the gases before the jet tube. So the whole Mason jar was effectively a bomb. The explosion blew the cork out of the sealer, while simultaneously blowing the sealer through the ring AND the ring off the threads of the jar. The jet tube shattered as it hit the ceiling.
Fortunately, I wasn’t hurt. And I don’t even have a book to blame for the mishap – just my own interests and inclinations, friends who were all interested in this kind of thing, science teachers who introduced me to fascinating things, and parents who encouraged my interest in science and tech.
That was only one of several close calls I had while playing with things that go ‘boom’. The last one, when I was in Grade 11, finally gave me good sense without injuring me or my family, and I quit doing that kind of stuff.
I remember that book. Interesting reading and very interesting to a high schooler who was already reading above his grade level. One of his articles in the March 1968 issue on the subject of small transmitters, prompted me to find the book that they reviewed. I might even send a Tip on them.
I too was influenced by this book. I recently dusted it off and built a Wilson Cloud chamber using dry ice now available from Harris Teeter: More on my cloud chamber (with videos of the traces), and on C.L. Stong’s wonderful book here: https://soldersmoke.blogspot.com/search?q=Cloud+Chamber
We had a set of The Boy Mechanic books. I remember seeing an article about how to make your own glider airplane and thinking that would be fun.
.
Re: chemicals and home made explosives. Eventually you need nitric acid which is hard to come by as a young lad and harder (but not impossible) to make at home from first principles. So I put that idea on the shelf for a while and got a chemistry degree instead. Even getting “easy” stuff like a bit of cannon fuse was impossible. Maybe they sold it on the shelf next to the chewing gum in the 1940’s. Also, it turns out there was a whole established professional field for this stuff and they would actually teach you how to do it (somewhat) safely and give you the materials and lab equipment to boot.
.
After graduating, they actually paid me to do it! They still do! Chemistry is the best.
The Boy Mechanic Books are not to be slept on. I had a reprint set from Lindsay Publications.
The Stong book I found in an unassuming gray cover and it covers a wide range of scientific disciplines, much as the early Scientific American Magazine did. I have two fragile copies of SA from 61 and 62. The depth of the articles in that era* and the enthusiastic Space Race vibe to the ads are fascinating–
*one from John Bumpass Calhoun, Ethologist who created the Universe-25 rat experiment to study the long term effect on populations of crowded living, while removing the stressor of resource scarcity.