Once again, [Afroman] is here for you, this time breaking down electrolyte and the terminology behind batteries.
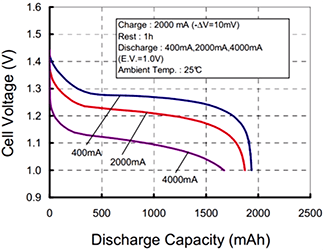
Volts and Amps are easy mode, but what about Amp hours? They’re not coulombs per second hours, because that wouldn’t make any sense. An Amp hour is a completely different unit podcast, where a 1Ah battery can supply one amp for one hour, or two amps for 30 minutes, or 500 mA for two hours.
Okay, what if you take two batteries and put them in series? That would double the voltage, but have the same Ah rating as a single cell. Does this mean there is the same amount of energy in two batteries as what is found in a single cell? No, so we need a new unit: the Watt hour. That’s Volts times Amp hours, or more incorrectly, one joule per second hour.
Now it’s a question of the number of cells in a battery. What’s the terminology for the number of cells? S. If there are three cells in a battery, that battery has a 3S rating. You would think that C would be the best letter of the alphabet to use for this metric, but C is entirely different. Nothing here makes any sense at all.
What is C? That’s related to the number of amps a battery can discharge safely. If a 20C battery can discharge 2200mAh, it can deliver a maximum current of 44 A, with 20C times 2.2Ah being 44A.
So there you go. A complete description of something you can’t use logic and inference to reason through. Video below.
Continue reading “A Description Of Maddening Battery Terminology”