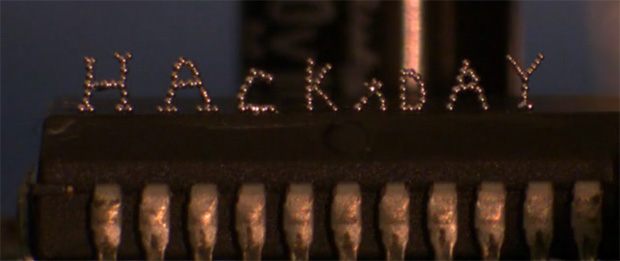
While 3D printers of today are basically limited to plastics and resins, the holy grail of desktop fabrication is printing with metal. While we won’t be printing out steel objects on a desktop printer just yet, [Collin Ladd], [Ju-Hee So], [John Muth], and [Michael D. Dickey] from North Carolina State University are slowly working up to that by printing objects with tiny spheres of liquid metal.
The medium the team is using for their metallic 3D prints is an alloy of 75% gallium and 25% indium. This alloy is liquid at room temperatures, but when exposed to an oxygen atmosphere, a very thin layer of oxide forms on a small metal bead squeezed out of a syringe. Tiny metal sphere by tiny metal sphere, the team can build up metallic objects out of this alloy, stacking the beads into just about any shape imaginable.
In addition to small metal spheres, [Collin] and his team were also able to create free-standing wires that are able to join electrical components. Yes, combined with a pick and place machine, a printer equipped with this technology could make true printed circuit boards.
Even though the team is only working on very small scales with gallium, they do believe this technology could be scaled up to print aluminum. A challenging endeavour, but something that would turn the plastic-squeezing 3D printers of today into something much more like the Star Trek replicators of tomorrow.
Video demo below, or check out [Collin]’s editing room floor and a vimeo channel. Here’s the paper if you’ve got a Wiley subscription.
[youtube=https://www.youtube.com/watch?v=ql3pXn8-sHA&w=580]














i love some of their wéirder extras.
Can someone pirate the paper please!
bp;dr
http://www.filedropper.com/advvm
Thanks a lot!
that may be the fake mercury that you can get from many scientific stores.
This is not mercury, it’s galinstan or gallium or GaIn :)
I don’t think you could make PCBs using this. Galinstan and similar room-temperature liquid alloys are *very* expensive.
They also tend to corrode/dissolve other metals. An interesting example of this is adding some galinstan to aluminum, which by partially dissolving the aluminum, continually breaks the integrity of the protective oxide layer. Without that, aluminum oxidizes so fast in water that it *burns* much like sodium metal, with copious hydrogen gas production.
Half true. It is expensive and chemically wonky.
It runs for about $70 per mL. A 1 cm wire is about ~0.1 microliters the way I did it.
The alloying properties of gallium can be pretty destructive, but our in-use work with gallium-copper meshed devices has been pretty stable over a year’s time. Some of my colleagues could better comment on this though!
How about Field’s metal? :)
Do i understand it right, the metal inside the blob is actually liquid and is only held in place by the oxide shell? The structures have no mechanical rigidity at all?
Some oxides are incredibly strong, such as aluminum oxide, nearly as hard as a diamond. This might look stupid when you realize there is liquid inside, but in reality this is a good start. The next step would be miniaturizing the balls to such a point that there is more surface area and less volume of liquid, which should increase the strength for all intents and purposes.
You got it. Nail on the head!
Sorry. Old moniker. I think I fixed it.
I like the setup with homemade parts, and I noticed you’ve got well over 0.025mm of precision with the needle, how was that achieved? A manual mill? Some old printers? RepRap?
And a followup: how was the ball droplet so precisely managed?
Our lead screw (pro equpment) for the stage was just that accurate. IIRC, the best I got with my home-brew home-depot bolt+nut+laser pointer assembly for the pump was 0.05-0.1 mm. Of course over really (cm) long distances, I’m sure you’d lose that accuracy.
Followup- analog pressure regulator at ~40 psi + a milisecond range solenoid valve. I’d played with pressure/time it until I got drop sizes I liked, and they stayed consistent until some environmental factor changed.
Bunch of noob questions:
1) What’s the upper bound? How big can you make a single ball before it’s own weight collapses the oxide shell? How thick a ‘wire’ can you build before it falls apart?
2) What is the maximum supported current/voltage for these “wires”? What about heat? Do they collapse after a certain temperature?
3) How many of these balls can you stack up vertically before the base collapses? Do they collapse if you shake the circuit board?
Would 3d printing a plastic support structure around them help?
1) Yknow, I don’t know. I suppose more than a few mm, but less than a cm wide. The wires can be infinitely wide… in zero gravity. In real life, your practical upper limit is mostly limited by the oxide strength and the internal pressure in the wire. If I had to slap a number on the diameter, I’d say 0.5 mm is the upper limit to practical wires, and 1 mm is the widest diameter I’ve ever gotten without calling what I just made a “blob” I can get more in depth about the internal Laplace pressure and whatnot if that doesn’t answer your question.
2) I’ve run an amp or two through a 0.5 mm wide x 0.5 cm long wire with no problems. Under heat, if anything the oxide should thicken (up to a point) and these wires would probably get stronger.
Fun fact: I microwaved a C shape wire. Of course, huge voltage built up at either end and vaporized the tips, but the wire mostly survived.
3) I like this question: Depends on a lot of factors that can’t be answered easily since the stacked droplets aren’t a perfect structure and it depends on your geometry (kinda like asking “how tall can you build a world of good tower?”). The droplet chains tend to behave like a gorilla tripod, but each joint is unequally strong depending on how much of the sphere surfaces “welded” In real life, the smaller you go, the stronger the stack gets (resistance to shaking the circuit board). My personal record = a 1 cm single freestanding wire 200 microns wide (think of getting a perfectly straight beard hair to stand on end)
THERE IS A THEORETICAL LIMIT HOWEVER based on the hydrostatic pressure and geometry, essentially you cant exceed a surface stress of ~0.7-0.8 N/m or the skin breaks and you’re done for. For the simplest geometry–a wire–that limit is (density*g*height of structure)/(circumference of wire)
1) So less than 1mm wide… Uneducated guess says that should be perfect for data.
2) And 1 amp? Awesome!
So heat increases the strength of the oxide shell… So you could make circuits, or metal structures that collapse or “self-destruct” if the temperature and/or amperage drops?
And here’s a weird one… is it possible to create a “tube” using the oxide shell? So create a ~1mm wide cylinder. Poke a ~0.5mm hole down the length without collapsing the shell? Probably wouldn’t be strong enough to support any liquids or gasses…
Aerosolizing the material with a controlled atmosphere, temperature, and precise timing would solve the oxide surface-area problem. Print speed would be rather…slow, though.
A babington burner can atomise/sputter copious amounts of fluid. See little reason why they couldn’t work with a suspension of metal in a solvent, or even a molten metal such as aluminium. Inert gas to power the atomiser. Resolution might be a bit coarse/somewhat fuzzy, but I think print speed could be made pretty high.
Would probably need to be printed vertically onto a surface that moves. Perhaps some kind of electrostatics could help focus the metal spray- like an electron gun.
Back when 3d printing was known as rapid prototyping, I once spoke with a man who had developed a “rapid prototyper.” He told a story of someone (may have been himself) who would make raw somewhat complicated parts by laying welds on top of each other over and over until he had the rough shape of the part he needed and would then mill it down to spec. That was his inspiration for the $100,000 3d printer that he was demonstrating for us circa 2005.
EBM – Electron Beam Melting
Woah, didn’t know about this. Thanks!
Perhaps a TIG head on an X-Y gantry could emulate EBM. Problem is the normal gas flow from a TIG might blow the powder away. Perhaps putting the entire machine in a sealed box, and filling the box with gas would work?
Stanford did work on this as “Shape Deposition Manufacturing”, but with alternating weld and machining steps so they got the additive manufacturing computational complexity instead of the subtractive – they could guarantee access for the cutter by not putting the bits it could collide with on until after the cut.
Is it possible to lay a slim contact of this on paper or a more stable material, much like drawing with a pencil?
Would be more reliable to use a corkscrew to control the flow of the material? I t would have to be very small… What is the viscosity?
1) Yes, papers have been published using a dip pen technique.
2) Viscosity is nil, around that of water. Incidentally, we are looking into the screw extruder on another project though. I like the way you think!
A few years ago I was involved in a similar project at university.
We had a system which printed low-melting-point solder in droplets of approx. 200µm diameter. The solder was placed in a glass tube which had a small steel piston inserted on top of the solder.
Around the tube two coils were placed which heated the solder and moved the piston to force the molten metal through a very thin nozzle.
The nozzle was formed beforehand by heating the glass to softness and blowing compressed air through the tube. This gave reproducible nozzles. We did no 3D-printing with it, just placing solder bumps for flip-chips.
Unfortunately the paper is behind a paywall (http://ieeexplore.ieee.org/xpl/login.jsp?tp=&arnumber=5723434&url=http%3A%2F%2Fieeexplore.ieee.org%2Fxpls%2Fabs_all.jsp%3Farnumber%3D5723434) but I might have the original PDF on my PC at home. If i find it, I can post it later today.
Ich würde zu sehen das mögen .
How big were your nozzles?
How about using something like Rose’s metal (or woods metal, if you don’t mind the cadmium) in the same manner as all the filament extruders on hobby 3D printers work?
It should be a lot more rigid and would be perfect for making lost molds for casting aluminium (or other materials)…
There’s also great potential to increase the “RepRap-ability” of…RepRaps… :D
Field’s metal? ;)
Why not try this with solder?
Exactly my thought. Or tin.
If we do anything outside of room temperature, we want to jump up to stronger metals to make it worth the trouble.
You would have to heat the solder. I was just looking up the melting point of copper.(1,948 F) It seems a bit steep.
Yeah there are a couple reasons for not using something higher melthing, but the short one is “start easy”.
wouldn’t adding different ratios of the materials and or impurities change the properties to something slightly more rigid or fluid at a given (say ROOM) temperature or perhaps more or less resistive. Come to think of it i wish i knew about this stuff before now.
Sure would. I’m not solid state chemist, but I wonder if adding certain dopants to the bulk metal might make the oxide p or n type semiconductor upon oxidation = soft transistors.
Now you’ve gone and said it. Print your own ICs. Sure, they’d be bigger than the ones you can buy, but you could print circuits in semiconductors possibly as easily as making circuit boards.
What does the melting point of copper have to do with solder (400-900 F) ??
yeah, I’m confused also… solder melts at what seems a pretty reasonable temp. to work with….think soldersucker in reverse…maybe i’ll have to try it myself with my homemade CNC mill…
So…. don’t these melt at.. ~ 100 degrees F?
60.2 F
Liquid metal? WTH is that supposed to mean?
-A mimetic poly-alloy
I think it would be a nice “give it a try” to feed an reprap with a roll of 1.5mm solder (the one without flux inlay) and fire it up with an simple model *g
if i had one, i would give it a try :)
Is it automated or directed by hand? Kinda looks controlled by hand to me in the video…
Hand controlled. I automated at one point but the equipment is so old we cant even control multiple axes at once.
Make your own fusible alloy
An easy fusible alloy can be made from bismuth, lead, and tin.
Lead is toxic, so this alloy should be handled with care, and should not be used near food, and should not be considered a toy. For toy purposes, use Field’s Metal instead.
Bismuth can be found at sporting goods stores in the form of shot for use in shotguns. It is preferred to lead shot because it is not toxic, and doesn’t pollute the water where duck hunters shoot.
Because some of our readers were having a hard time finding bismuth, (especially outside of the United States), we supply it in our catalog.
Lead and tin are not hard to find separately, but they are particularly easy to find together, in the form of solder.
For our purposes, we will be using a slightly less common form of solder. Solder is usually made in the eutectic proportions, or in the nearly eutectic form of 60% tin, 40% lead. But some solder is available in the opposite ratio — 60% lead, and 40% tin. This is the form we need (unless you can obtain your lead and tin as separate items).
The eutectic form of the bismuth-lead-tin alloy is 52.53% bismuth, 32.55% lead, and 14.92% tin, by weight. The compound is Bi8Pb5Sn4.
If you have the separate metals, you can weigh them out and melt them together, and you will have an alloy that melts at 203° Fahrenheit (95° Celsius).
If you have the 40/60 tin/lead solder, you can weigh out equal parts of the solder and the bismuth, and melt them together. This gives you a mixture that is not eutectic, so the melting point is a range from 203° F (95° C) to 219° F (103.8° C). This will still melt in near boiling water.
source : http://sci-toys.com/scitoys/scitoys/thermo/thermo4.html : Simon Quellen Fields
Nice video ;-)
Might also work with 8% Ga 75% In and the rest tin and copper.
You can do some really interesting stuff with this liquid, our lab dissolves iron particles into it to create ferromagnetic fluids. However, it has a downside. It will destroy nearly everything it comes in contact with except for stainless steel and titanium.
Our lab tried this a while back, how’d you get the iron in the EGaIn?
We create a solution of 23% Indium and 77% Gallium. Use HCl to break down the oxide layer that forms on the surface, then drop very small iron particles in there (~5 micron), it can hold nearly 30% iron by weight. It’s not fully stable, a strong magnet (near a tesla) will rip some of the iron out, also some settles to the ground when you create high concentrations.
Also, for reasons unknown, not all of our iron powders have worked. ‘Identical’ batches have failed. Oxidation is a major problem, even the iron inside the solution rusts. We’re dealing with this by leaving our samples covered with 0.1mol HCl, but an inert atmosphere would be ideal.
I’m waiting for the day were Direct Metal Laser Sintering machine are available for cheap (by cheap, i mean much less than $100,000. although 100k is probably dirt cheap of a DMLS machine today). print part -> dust off (put waste powder into reuse bin, i’d assume) -> little extra machining and quality control -> put into final product.
To anyone that doubts 3d printed metal, look up what GE and Pratt has been stating about additive processes. http://www.cnn.com/2013/06/11/travel/leap-engine-3-d-printing and http://www.sae.org/mags/aem/12061/
The main reason I was looking at copper was because of the low resistance. If laid think enough, the type of metal you’re using comes into play. I don’t expect to be running 10+ amps, But my primary thought was layering with many pcb for more compact home made items. Tin has 10X the resistance of copper, just something to think about…
Thin**
10-20x resistance, um back of envelope horrible math, = 3-4x wider wires, it isn’t horrible, but not great industrially.
This is absolutely fantastic tech – but I have to point out it does lend strong weight to the argument that 3d printed guns *will* one day be actually usable without blowing your arm off :-/
I want tech advancement, I want my Ultimaker to slower evolve to a star trek replicator…
but I also don’t want any drunk,nutter or child able to just print off weaponry.
I also don’t want censorship either though – weapon censoring will almost certainly be a excuse for more general IP censoring.
I hate to be the downer guy, but I really think 10 years down the road, if not less, we are going to have problems.
*slowly evolve, I meant to say
You want a lot of things. I’ll settle for one thing. Personal responsibility.
I wish you people would F off with the “3D printer == Star Trek Replicator” stupidity. It. Will. Never. Be.
Why don’t people try using lead free solder as a filament?
Why don’t people try using lead free solder as filament?
Just talked with my boss today. We might, although I want to go for the insane goal of using aluminum.
large syringe full of solder paste, leadscrew on piston, and a heated tip….solder paste is made of tiny balls, if you use the right sized orifice you should be able to get some tiny melted balls in a controlled manner… I think silver solder paste is available also…
We have a pretty cool amalgam slurry that doesn’t use Hg in place of solder paste in your idea. But yeah, we’re on it.
Just thought I’d throw it out there that if you are in the Raleigh area, grad student or not, we are looking for a successor to the project (I’m moving). If you’re interested. Hit me up at caladd2@ncsu.edu.
Interesting idea, make the iron particles with a SiO2 coating and coat that with tin using SnCl2 vapour.
That ought to fix the problem :-)