Tritium, or 3H is an isotope of hydrogen which has been used as everything from radiolabel in analytical chemistry to a booster to kickstart the chain reaction of nuclear weapons. Lately tritium’s most common use has been in key chains and jewelry. A small amount of tritium is stored in a phosphor coated glass tube. The beta decay of the tritium causes the phosphor to glow. The entire device is called a Gaseous Tritium Light Source (GTLS).
In the USA, GTLS devices are only allowed to be used in specific cases such as watches, compasses, and gun sights (MURICA!). Key chains and jewelry are considered frivolous uses and are prohibited by the nuclear regulatory commission. Of course, you can still order them from overseas websites.
The safety of GLTS devices have been hotly debated on the internet for years. They’re generally safe, unless you break the glass. That said, we’re happy getting our radiation exposure through cool hacks, rather than carrying a low-level source around in our pockets.
Enter [Ted Yapo], an amateur astronomer. After tripping over his telescope tripod one time too many, he decided to take matters into his own hands. He’s designing TritiLED, a dim LED light source which can last for years. [Ted] is using a Luxeon Z LED, driven with PWM by a PIC 12F508 8 bit microcontroller. Running at 26.3 μA, he estimates about a year of run time on a CR2032 watch battery, or a whopping 15 years on a pair of lithium AA cells. Sure he could have done it with a 555 timer, but using a micro means more features are just a few lines of code away. [Ted] took advantage of this by adding a high brightness mode, blink modes, and an exponential decay mode, which emulates the decay of GLTSs.
Best of all it’s all open source. [Ted] is publishing under the (CC-BY-SA) license on Hackaday.io.

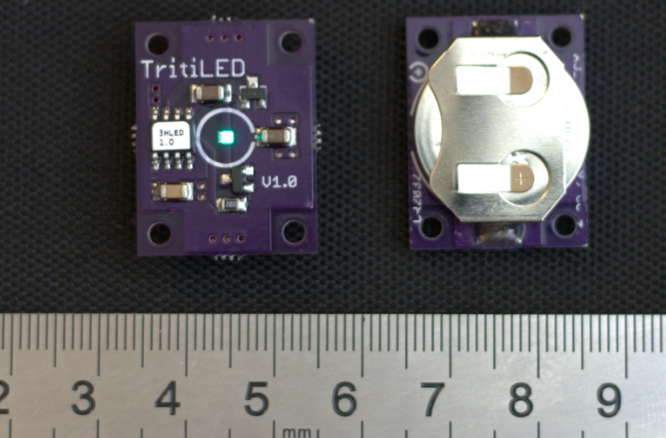














pretty neat. you’d think an astronomer would’ve used a red LED though but I reckon he was going for an authentic tritium look.
should’ve clicked the link before I posted, there’s all kinds of purty colors
Why would you use a red LED?
For the same amount of power, the human eye is far more sensitive to green then it is to red (or blue)…
Red is used because it doesn’t kill your night vision.
https://en.wikipedia.org/wiki/Purkinje_effect#Use_of_red_lights
That makes no sense whatsoever, why would you want night vision in a submarine? Just give people enough white light so they can see what they need to see…
The water is really dark and they like to look at the pretty fishes.
The bright lights would scare away the pretty fishes.
https://www.quora.com/Why-are-submarine-operating-rooms-kept-dark
In case you have to look out the periscope?
Well you also want to maintain a decent circadian rhythm so that your crew doesn’t feel like shit all the time. White light at night is a great way to screw up that rhythm. I use red lights at home, as well as f.lux/twilight, and my eyes and sleep cycles are so much better off because of it (yes, it’s subjective, but it feels better than staring into blinding white light all the time).
If you ever watched movies with submarines you’d know they can switch to red light for when they surface at night and want to not ruin their night vision or to be easily spotted. It’s not a case of always having red light since that would cause people to go mad I expect.
Wow. This.
I’m probably going to make a few – one day I hope the design collapses down to the size of a small/medium electrolytic and is powered by one, too :)
Also – charge by induction and put constellations in the ceiling. Wave your coil and watch them all spring to life!
Yes! Add a coil and a supercap and put these everywhere!
There’s probably enough power in wifi signals for it.
Worth a try, but astronomy amateurs also like to go out to remote areas to escape light pollution and would be in places with low WiFi presence. But maybe cell tower emission can be tapped? Those are more widespread, albeit not covering all situations.
I like the idea. Guess the only problem is that shrinking it’s size even more and at the same time adding an inductive charger is very hard. I’m not all too deep into RF power transmission, but i would guess the larger your coil surface the easyer it is to get energy out of the field, and of course, the weaker the field (distance to transmitting coil) the bigger you would want your coupling surface.
You could just use smaller coin cells, like the CR1220, but you will loose quite some run time (CR1220 has around 40mAh, the CR2032 has around 220mAh).
The idea with supercaps could work pretty well even without the wireless power part, problem is that supercaps are not that magical and still need volume to store energy, and depending on how much run time you want to get from your LED, the cap can quickly get even bigger than your CR2032 and large supercaps get quite expensive very quickly…
I’m curious as to what could be done to reduce the part count to the absolute minimum: how low can you go?
Uhm. 3.
Battery, resistor, LED :p
Battery, led. The battery is carefully oxidized on surface to obtain desired resistance.
Wouldn’t have the same lifetime. Human eyes perceive LED brightness non-intuitively; if you run the LED with PWM you can get the same perceived brightness even though the LED is only on 1% of the time. That’s a big win for battery life over running it continuously at lower current.
You are misunderstanding the human eye visual response (and in fact are getting it opposite to how eyes actually respond to brightness).
LEDs are non-linear and dimmer than they should be at very low currents because of recombination current that acts like a large resistor in parallel with the device. Run ’em at higher current and a larger fraction of that goes into making light. Nothing to do with your eyes.
Minimum parts count self oscillating flyback converter.
1x LED obviously
1x Transistor (selected for specific Base-Emitter leakage capacitance)
1x torodal transformer with windings designed for specific resistance, inductance & ratio (usually 1:1).
Some copper tape or similar for RF shielding, or not.
If designed well, will run LED for years on any single cell of more than 0.7v, with additional feature of telling you when battery is nearly dead by flashing LED as cell voltage dives below BE forward bias and recovers.
You may add:
Capacitor if transistor leakage capacitance insufficient
Variable resistor for frequency / brightness control
Possible circuit ideas at: http://www.talkingelectronics.com/projects/LEDTorchCircuits/LEDTorchCircuits-P1.html
Could he have used a 555? My understanding is they are pretty current-hungry little devices. Set and reset are just voltages on a voltage divider that wastes a fair amount of power..
A voltage divider easily can be in 10s of kiloohms, if the device is CMOS, you could probably supply the required trigger current with an even bigger resistance…
Yes, it can – or better “could” be and it would still be ~100µA of current. And the classic 555 has a divider of 3*5kOhm. But I would just have used a resistor.
yes they can’t use it. to much current, also in the low current version.
Light switches are an ideal application for this sort of thing. I have a couple of tritium Traser Glowrings I bought some 15 years ago, they’re still glowing but obviously not as bright as they once did. I ended up putting them either side of a light switch so when coming in at night I can always easily find it in the dark.
Even if you break the glass vial inside those Tritium lights, they are not that dangerous. Tritium is a gas whitch dilutes pretty quickly with the air. I’m not saying you should inhale a broken light, but open a window and you’ll probably survive.
Carrying two of these ringlights in your pockets for 50 years has a similiar dose of radioactivity as one intercontinental flight. I have one on my key chain for about 5 years now and I’m still alive.
Or put another way, you’ll pick up more of a radioactive dose by eating a banana.
Well, that is an understatement. No ionizing radiation will get out of any properly sealed container of Tritium. Tritium emits beta radiation with an energy of 19 keV (http://crete.homeip.net/show_nuclide/10003/). This gives it range of 1 cm in air or less than 0.5 mm in glass (http://physics.nist.gov/PhysRefData/Star/Text/ESTAR.html). In fact, most radiation detection instruments can not detect radiation from Tritium as it is stopped in the outer shell of the detector.
Furthermore, I do not know of any cases where tritium has caused acute radiation sickness (even after ingesting large amounts) as the energy of the radiation is so low. There are far nastier radionuclides.
While it’s a pretty neat light source, I have to ask why this solution to his tripping problems? I do some amateur astronomy and astrophotography and of course have run into similar issues of not wanting to trip over my tripods. My solution was to go to Walmart, buy some glow in the dark duck tape and put it at the top, bottom and adjustment knobs/handle of my tripod. Don’t ever have to worry about charging them since my tripods are almost always stored in my vehicle, too many trees around my house for star gazing. Just seems like way overkill for a very simple problem.
After many years lurking HAD I found out there’s really no overkill in anything ;)
Specially when the goal is to light a led
You have to do something on cloudy nights…
Neat Hack to get use of the µC would be to use the LED as Photodiode in dark-times of PWM, so that it could be switched of on high ambient light. This would further increase run-time to several years if it is stored in daylight.
yes correctly, an LED can be used to measure light level, and this way stop the light in case it is day.
just too bad the idle current is actually way too high to give anything near the claimed run times,
I tried exactly the same thing, but took it even futher, only an ultra short blink every 2 sec, but the internal leak current of batteries and just their storage time with nothing connected at all, is about the same, as what such “bugs” draw. I got very disapointing run times, 8 month with 2 AA alcaline cells
Hence the CR2032. I understand that its self-discharge rate is a lot lower than that of AA alkalines.
Back in the 70s, TI used to make an IC that did exactly this function. It was used in things like emergency flashlights on airplanes so that you could find them in the dark by the blinking LED.
Today’s Alkaline cells have expiration dates at least five years after manufacture, and I would guess this is to about half their capacity, so if your bugs are discharging them in 8 months, it’s not due to self-discharge of the batteries. You need to look at ALL of the current paths in your circuit. Could be that the light level sensing circuitry is taking more current then the LED would!
So, after all the talk of tritium this is just a dim LED? Dull.
*This* is the sort of (bio)hack that should be on here.
http://forum.biohack.me/discussion/1470/firefly-tattoos
Warning: the safety police contingent may get a little overexcited.
SAFETY POLICE:
Don’t put it in yo veins or arteries.
Especially that 20mm needle :D
damn it, wanted to write 3mm :(
Why not use a resitor, battery and LED and get even better runtime???
Because LEDs are really crappy and inefficient at very low currents, behaving like there’s a (large and non-linear) resistor in parallel with the LED: at low currents most of the power is going into that parasitic resistor (recombination current) and not into light. Pulse it at 1% duty and 100x the current (i.e., same average current) and you get FAR more light out. (Note well: the opposite happens at high currents). They have got much better: Cheap (such as they were) LEDs in the 70s barely lit at all at 1 mA, ran well at 10-15 mA (and died at 30 mA…).
I tried exactly this – here’s a picture of two LEDs run with 26uA battery current – one at DC and one with 3us 100mA pulses:
https://hackaday.io/project/11864-tritiled/log/40428-electrical-efficiency-measurements
I actually have to crank the DC current up to 75-80uA before the LEDs “look the same.” @Paul: thanks for the explanation! You can read in that log linked above where I was scratching my head about why the LED is so lousy in the uA current range. You commonly see the top end of the relative brightness vs current curve in the datasheets, where the LED is more efficient at moderate currents than high ones, but I’ve never seen the curve all the way down to zero. Do you have a reference for more details?
The camera actually doesn’t capture how much brighter the pulsed LED appears to me. I suspect there is a physiological component to this, where the pulsing appears brighter. Here, they say that green LEDs pulsed around 60Hz look 2x brighter to the eye:
http://techon.nikkeibp.co.jp/english/NEWS_EN/20080407/150114/
Using the 18ms nominal watchdog timer for pulse generation, you get 55Hz pulses with this circuit. Close on frequency, but with much shorter pulse width than in that reference. More study is required…
The only one I could find with a useful X axis (i.e. log scale X) is this article about LED droop
just searching google images for “led droop” finds tons of instances of the high current end of things
I saw that in your log — sorry I neglected to mention it above.
Here’s an example: http://optoelectronics.liteon.com/upload/download/DS22-2000-230/S_110_LTST-C191TBKT.pdf Figure 4 shows the low end dropping off very fast below a few mA.
That Shimizu paper from Nikkei claims the “pulsed LEDs are brighter” effect is due to the Broca-Sulzer effect, which is horseshit — the Broca-Sulzer effect, though totally real, happens only with relatively long pulses (dozens of milliseconds). (see, for example, http://webvision.med.utah.edu/book/part-viii-gabac-receptors/temporal-resolution/ ) It absolutely isn’t an effect on the sub-millisecond pulse lengths. What Shimizu is seeing is the poor efficiency of the LED driven at low current, and higher efficiency when run at 20x the current but 5% duty.
A previous comment I made hasn’t appeared yet. Maybe it landed in a moderation queue because of a bad word I used. In any case, here’s another example of low LED output at low currents:
https://www.researchgate.net/figure/236974286_fig1_Current-and-light-output-intensity-vs-voltage-characteristics-of-the-GaN-LED-with-size-of (see the last two figures)
Really happy to see that you have considered LED droop in your project. :)
Though a great tool, unfortunately this device is useless for one of the best uses of a tritium light in astronomy: camera calibration. A tritium light source (e.g. from http://www.betalight.nl/ ), is an ultra-stable, zero-noise low-intensity narrowband light source ideal for testing and calibrating CCD camera sensors: https://www.google.com/search?q=betalight+ccd+testing
But, will the NRC let me buy one for that purpose? Doubtful.
Well, you could fill out the request form at http://www.betalight.nl/en/contact/how-to-order.html to find out. Their customer list shows many USA-based customers (I assume you’re in the USA from your question). Or you could just order one off Amazon – they even have some with Prime service — you could have it tomorrow.
BTW: Truly excellent hackaday.io writeup. Exemplary work. I’m sure I speak for a lot of people here in saying we appreciate that kind of extra effort to make an accessible, accurate and thorough writeup.
A really small solar cell, a supercap, and this will last forever (nearly).
I did that long ago to challenge the ATTiny10
How about just buying a cheap $25 tritium pistol front sight and put it on the expensive telescope?
https://www.amazon.com/Meprolight-Tru-Dot-ML10222-ML10224-ML10226/dp/B005AUD01G/
I guess I don’t understand why a battery-powered LED is better than tritium, which makes for a lot smaller package.
Because hack.
Good idea for me though, I want a bulk-pack.
If only they were cheaper…
You can buy tritium in glass capsules (blocks beta decay??). Look at the size and cost of this one!
https://www.amazon.com/Vials-Tritium-Self-luminous-15-Years-5x80mm/dp/B010EZQ50Y/ref=sr_1_21?ie=UTF8&qid=1468347285&sr=8-21&keywords=tritium+vial
If you replace the circuit with a microcontroller, key and other parts with only one resistor, it will be a great hack! If you do the opposite?
Oh, the author made an excellent trolling for the readers of the HaD. I laughed for a long time!
I build one with a 10k resistor, cr2032 battery and a white led, two years running since jan 2018, still running