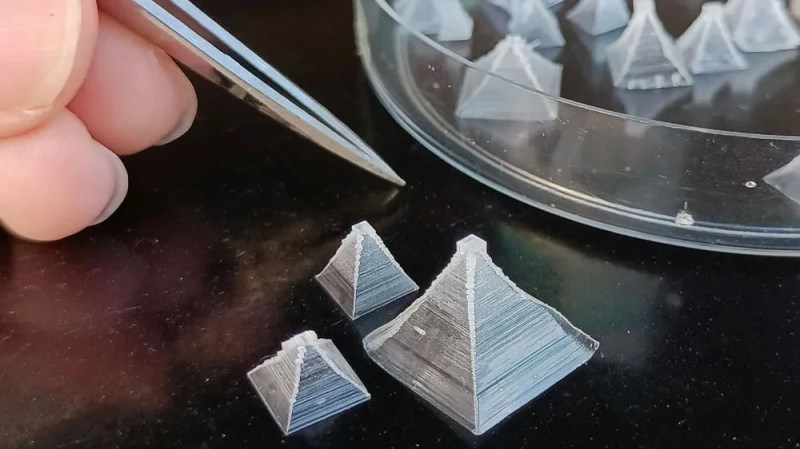
The regular granular table salt you’re used to isn’t the most attractive-looking seasoning out there, even given its fundamentally compelling flavor. You don’t have to settle for boring old salt anymore though, because [Chase] has shown us you can grow your own pyramid salt crystals at home!
Pyramid salt crystals can grow naturally, and typically occur in locations where salt pools are undisturbed under the warmth of the sun. However, it’s possible to grow them on purpose, too. As a bonus, their hollow structure means they dissolve very quickly on the tongue, and can taste “saltier” than typical granular salt.
 To grow your own, you’ll need a bag of salt, which is mixed with some water. You’ll want to do so in a glass dish, as the salty solution you’ll be making can ruin metal cookware. The dish can then be heated up on an electric hotplate, which is used to heat the solution to between 60 and 70°C.
To grow your own, you’ll need a bag of salt, which is mixed with some water. You’ll want to do so in a glass dish, as the salty solution you’ll be making can ruin metal cookware. The dish can then be heated up on an electric hotplate, which is used to heat the solution to between 60 and 70°C.
A small amount of food-grade potassium alum is also added to the solution to calm the convection currents in the heated solution, allowing the crystals to form gently without sticking and clumping together. As the water boils away, the rectangular-pyramidal crystals grow.
Naturally, you must be careful before eating the results of any home-grown lab experiments. However, [Chase] reports having licked some of the crystals and has confirmed they do indeed taste salty. [Chase] also notes several ways in which the parameters can be changed to grow different types of pyramid crystals, too.
We’ve featured [Chase]’s crystal-growing work before. If you’ve got your own cool DIY crystal projects cooking up in the lab, be sure to let us know!













Obviously, heated water can dissolve more salt. But I wonder…if you mixed a saturated solution of salt and water at room temperature, then placed the growth medium in a vessel and placed it under a vacuum (“boiling” away the water), would the outcome be the same?
Different environmental conditions lead to different outcomes. The fast evaporation from your hypothetical setup would probably lead to small cubic crystals…
The secret sauce for the pyramid configuration seems to be the specific temperature of the water and the addition of potassium alum to prevent convection currents.
Well… that’s kind of my point, though. Application of the vacuum need not be abrupt…it could be gradual. The absence of a temperature gradient would preclude convection.
Wouldn’t lowering the pressure in a vacuum cause way more turbulence with low temperature boiling?
Not if you are careful about how much vacuum you pull. You don’t have to pull a hard enough vacuum to cause boiling to significantly increase the rate of evaporation. It might take some trial and error to work out the correct pressure, but you could probably calculate what you would need under ideal conditions and then adjust from there. A phase diagram for water would be a good starting point. Find your current temperature, and find the pressure where water starts boiling at that temperature. Then pull a vacuum to just above that pressure, and if it starts boiling, increase the pressure until it stops.
It might just turn out that the equivalent temperature for that pressure causes the exact same effect. So if 60-70C produces certain results at sea level air pressure, maybe a pressure that makes water boil at 50C would have the same effects at 10-20C. Alternatively, maybe the pressure difference would cause the salt to crystallize completely differently in that temperature range.
Someone should really experiment with this, because now I want to know! Wish I had the stuff to do it myself, but I don’t right now. (Also, maybe someone needs to do the same kind of experimentation but in a pressure pot, to see what increasing the pressure does… I mean, what happens if you heat it to 120C, under enough pressure to prevent it from boiling? Only, that might create a problem with humidity increasing inside the pressure pot, so you would need some kind of desiccant in there to remove the water vapor from the air as it evaporates…)
Not a whole lot more. At 20C the solubility of sodium chloride is about 36 g per 100 g water. At 100C it’s 38 g per 100 g water.
Changing the evaporation rate could affect the crystallization though, regardless of saturation.
From these very forums, this chemist was very surprised to learn that for NaCl, the solubility doesn’t change much at all with temperature of the solvent. It dissolves a whole lot quicker if the solvent is warmer though.
Is going have a different outcome as well
One of these days, I’m going to get/make a vacuum chamber. This is now on my list of things to try with it!
Salt crystals taste salty! Film at 11!
And unlike the other pyramids, no aliens were involved. :-)
How do you know? Lol
How do you know?
In which universe does water boil at 60°C?
I think author used poor choice of word. Water evaporates at any temperature, the higher the heat the faster the rate of evaporation. Boiling starts at 100C
Although water can boil at lower setting at higher altitude.
Technically below freezing it sublimes (evaporation is technically only liquid to gas, not solid to gas), but it’s basically the same thing.
A universe where the air pressure is around 3 psi
It boils at 68C on top of Everest, here in our universe.
From the thumbnail I thought this was going to be about some novel 3d printing. I guess in a way it is.
Someone tell Adam Regusea
To be fair, I have seen pyramid shaped salt flakes sold at the local grocery store where I live.
I guess they might use a similar process to make them at an industrial scale. However, they don’t seem to aim for as impressive sizes.