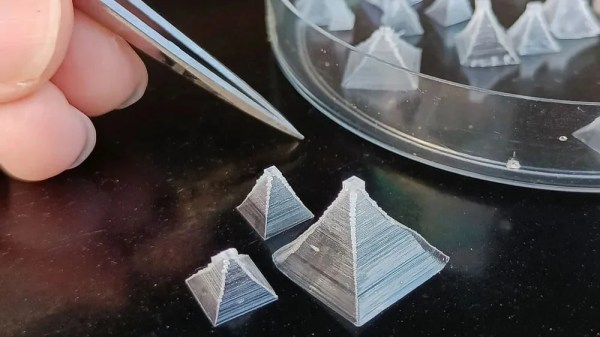
The regular granular table salt you’re used to isn’t the most attractive-looking seasoning out there, even given its fundamentally compelling flavor. You don’t have to settle for boring old salt anymore though, because [Chase] has shown us you can grow your own pyramid salt crystals at home!
Pyramid salt crystals can grow naturally, and typically occur in locations where salt pools are undisturbed under the warmth of the sun. However, it’s possible to grow them on purpose, too. As a bonus, their hollow structure means they dissolve very quickly on the tongue, and can taste “saltier” than typical granular salt.
 To grow your own, you’ll need a bag of salt, which is mixed with some water. You’ll want to do so in a glass dish, as the salty solution you’ll be making can ruin metal cookware. The dish can then be heated up on an electric hotplate, which is used to heat the solution to between 60 and 70°C.
To grow your own, you’ll need a bag of salt, which is mixed with some water. You’ll want to do so in a glass dish, as the salty solution you’ll be making can ruin metal cookware. The dish can then be heated up on an electric hotplate, which is used to heat the solution to between 60 and 70°C.
A small amount of food-grade potassium alum is also added to the solution to calm the convection currents in the heated solution, allowing the crystals to form gently without sticking and clumping together. As the water boils away, the rectangular-pyramidal crystals grow.
Naturally, you must be careful before eating the results of any home-grown lab experiments. However, [Chase] reports having licked some of the crystals and has confirmed they do indeed taste salty. [Chase] also notes several ways in which the parameters can be changed to grow different types of pyramid crystals, too.
We’ve featured [Chase]’s crystal-growing work before. If you’ve got your own cool DIY crystal projects cooking up in the lab, be sure to let us know!