It’s often said that the best science experiments are the ones which do not require any special devices or ingredients, which makes the use of what naturally comes out of one’s body clearly one of the winners. It’s also the beginning of yet another [Hyperspace Pirate] chemistry video that’s both fascinating and unforgettable — this time introducing a considerable collection of urine, and the many uses of the urea in it, including its use for refrigeration.
As icky as this may sound, it doesn’t even rank in the top ten of quaint things people have historically done with urine, so extracting urea from it is rather benign. This is performed by adding sodium hydroxide to the starting component after heating, which creates gaseous ammonia (NH3) which was then condensed into its liquid (dissolved) form. In order to create the target compound – being ammonium nitrate – nitric acid (HNO3) had to be created first.
For this the older, but cheaper and easier Birkeland-Eyde process was used. This uses high-voltage electrical arcs to break down the nitrogen and oxygen in the air and cause the formation of nitric oxide (NO), that subsequently reacts with atmospheric oxygen to form nitrogen dioxide (NO2). Running the NO2 through water then creates the desired HNO3, which can be combined with the ammonia solution to create ammonium nitrate. The resulting solution was then evaporated into solid ammonium nitrate, to use it in an aluminium cooling cylinder, with freshly added water.
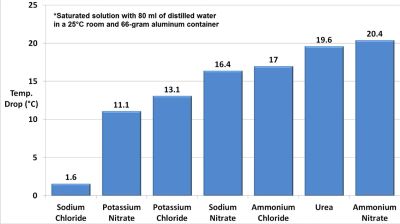
This is the simplest way to use the cooling effect of such solutions (pictured), but the benefit of ammonium nitrate over the original urea seems minimal. The low efficiency of this cooling approach means that the next use of urine will involve a much more efficient vapor-absorption cycle, which we’re sure everyone is squeezing their legs together for in anticipation.
We’ve been covering the refrigeration experiments [Hyperspace Pirate] has been conducting for some time now. If you’re into the science of making things cold check out how seashells can be turned into dry ice, or what goes into building a home cryocooler.
















Defcon Beverage Cooling Contraption Contest?
Literally taking the p**s!
Next describe the home manufacture of diesel fuel.
Back in the day, people used oil meant for pommes frites to power their cars. Rapeseed oil, more precisely. It wasn’t really legal, though, I heard. Not every diesel motor could handle it, either.
Possible to use ‘adblue’ perhaps? That’s urea and deionised water, and while not as cheap as natural sources, maybe has less of the “oh god its all over my bench” factor.
I would almost rather have urine on my desk than DEF/Adblue it always an almost impossible to fully clean up mess. In my Passat the DEF filler was in the trunk and only a few drops of vw adblue was enough to start growing crystals in the carpet. I don’t think I ever got it fully clean again.
Adding urine to compost gives the aerobic bacteria the additional nitrogen needed to break down cellulose.
It’s got what plants crave?
From Wikipedia:
“(Ammonium Nitrate’s) other major use is as a component of explosive mixtures used in mining, quarrying, and civil construction. It is the major constituent of ANFO, a popular industrial explosive which accounts for 80% of explosives used in North America; similar formulations have been used in improvised explosive devices.”
And you can still buy a 25lb bag at the local home improvement box store or garden supply. Heaven forbid you also get a gallon of MEK, which seems to include the local NARC attention
Gotta make sure you’re not a smurf
I’d have a hard time finding a household chemical that *can’t* be used for anything harmful. Plus, as far as explosives go, historically the ones that are used the most industrially aren’t the ones that are the most sensitive or powerful or any such thing – they’re the ones that get the job done with acceptable cost and safety.
I’ve not attempted to use the stuff, but if you read further on Wikipedia, you see that ammonium nitrate is often just considered an oxidizer which when given a secondary explosive can be convinced to detonate, consuming a bit of fuel and releasing the energy. You can sensitize ammonium nitrate if you want it to respond to high velocity impacts, but it takes multiple additives in the common commercial products which do that; else a typical blasting cap isn’t enough to set it off. It’s hygroscopic and doesn’t work when wet, so there’s a very common substance that can make it safer if there’s a problem.
It was primary charge for the Oklahoma city bombing.