A team at MIT led by [Professor Douglas Hart] has discovered a new, potentially revelatory method for the generation of hydrogen. Using seawater, pure aluminum, and components from coffee grounds, the team was able to generate hydrogen at a not insignificant rate, getting the vast majority of the theoretical yield of hydrogen from the seawater/aluminum mixture. Though the process does use indium and gallium, rare and expensive materials, the process is so far able to recover 90% of the indium-gallium used which can then be recycled into the next batch. Aluminum holds twice as much energy as diesel, and 40x that of Li-Ion batteries. So finding a way to harness that energy could have a huge impact on the amount of fossil fuels burned by humans!
Pure, unoxidized aluminum reacts directly with water to create hydrogen, as well as aluminum oxyhydroxide and aluminum hydroxide. However, any aluminum that has had contact with atmospheric air immediately gets a coating of hard, unreactive aluminum oxide, which does not react in the same way. Another issue is that seawater significantly slows the reaction with pure aluminum. The researchers found that the indium-gallium mix was able to not only allow the reaction to proceed by creating an interface for the water and pure aluminum to react but also coating the aluminum pellets to prevent further oxidization. This worked well, but the resulting reaction was very slow.
Apparently “on a lark” they added coffee grounds. Caffeine had already been known to act as a chelating agent for both aluminum and gallium, and the addition of coffee grounds increased the reaction rate by a huge margin, to the point where it matched the reaction rate of pure aluminum in deionized, pure water. Even with wildly varying concentrations of caffeine, the reaction rate stayed high, and the researchers wanted to find out specifically which part of the caffeine molecule was responsible. It turned out to be imidazole, which is a readily available organic compound. The issue was balancing the amount of caffeine or imidazole added versus the gallium-indium recovery rate — too much caffeine or imidazole would drastically reduce the recoverable amount of gallium-indium.
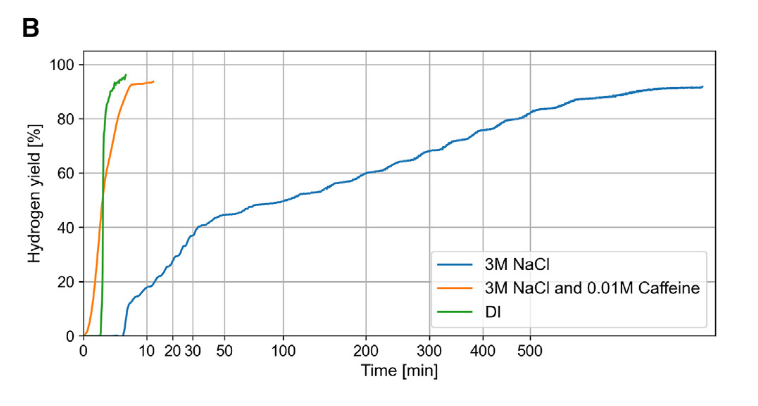
After some experimentation, they hit a magic number: a 0.02M concentration of imidazole resulted in consistent recovery rates of ~90% of the gallium-indium, which is comparable to the recovery rate in seawater with no catalysts of any kind! This method of hydrogen generation could make marine applications of hydrogen engines much more viable. By only needing to carry aluminum, imidazole and gallium-indium, the safety issues with liquid or compressed hydrogen disappear. This could make marine vehicles cleaner and more efficient while reducing the safety issues already present in carrying diesel or other marine fuels aboard.
The study goes into much, much more detail, so if you want to learn more, be sure to check it out! Thankfully, it’s hosted in an open-access journal so the knowledge is free for all to learn from.
[Thanks to zoobab for the tip, via ScienceDaily!]
















Man who knew my coffee habit could be so useful?
I’m puzzled: how much energy does it take to produce that aluminum in the first place?
It’s an almost zero sum game. The byproduct of the reaction goes back into the aluminium smelters and gets used to generate again pure aluminium.
No, in this context you must take into account the energy needed to refine the aluminium to turn back the aluminium oxyde to aluminium.
And this is where this bright idea turn into a bellow average one.
I think the idea here is not the “generate” energy but to have a easy and efficient way of transport and store it.
Have an intermitted energy-overabundance by Solar and wind? Heat up the aluminum furnace and make some ingots. Store those easy for a later date when you need the energy and you “disolve” themwith seawater, gallium and coffeine and you have back like 90% of your energy. A europallet of Aluminum would hold a HUUUUGE amount of energy. Save, nonvolatile and easy to handle and transport.
I quarrel with the very idea that aluminum “holds” “energy”.
So we’ll be storing, using, moving hydrogen at around atmospheric pressure? Not sold on that.
You’ve got the wrong end of the stick – the aluminium isn’t a power source but essentially a battery. Aluminium is smelted using electricity, which is free when renewables are over-producing, and can be stored safely and indefinitely. It can also be smelted close to where renewables are produced, and then transported, which alleviates pressure on transmission lines.
Enough of this nonsense! We need to go back to burning whale oil. Consider:
Sunlight feeds plankton, plankton feeds whale, whale oil feeds industry, with a bonus of ambergris for Hollywood… from which to make perfume to cover the stench of the movies they produce.
The CO2 produced in burning this oil is no greater than CO2 removed by the plankton, so the process is carbon-neutral, solar powered, and ethical, so long as you use free-range whales.
All I need to prove the concept is a whaling vessel, a captain with an eye patch and peg-leg, and a multi-milion-dollar “research” grant.
Who’s with me?
You are definitely on to something there! :D
Ahab, you’ve got to let this whale obsession go!
The world users about 100 million barrels of oil per day, Google tells me there are about 1.5 million whales in the sea (regardless of species), and that each whale provides 25-40 barrels of oil.
If we assume the high end of that range, and assume a barrel of whale oil contains the same energy as a barrel of fossil oil, that would be about 60 million barrels of oil, enough for just over 14 hours, minus the oil you used to catch the whales.
I hate to spoil your party, but I don’t think it’s going to work.
Arrrrrrrrr me hearty!
Arrrrrrrrrr me hearty!
Part of Vermin’s platform for years now.
Vote Pony party!
Everybody gets a pony.
DOD and social security budget diverted to pony breeding.
They take all American’s guns, and give us better guns.
Pony and whale oil based economy.
Victory is inevitable!
But we can make it happen this year.
Vote Vermin Supreme!
This is especially directed to ‘in the pot states’.
e.g. CA’s electoral college votes are doomed to go to the biggest idiot.
So vote your beliefs.
there used to be enough Wales to supply all the oil needs of the world. Imagine what the oceans must have looked like back then.
As per (1), “[per-kilogram Al production] energy consumption has decreased to 12.3 kWh. This is well below the world average of 14.1 kWh and Hydro’s own average of 13.8 kWh.
We are also testing another variant of the new technology, which we hope will reduce energy consumption even further to 11.5 kWh per kilo of aluminium.”
In a combined heat + electricity generation scenario, (2) states Al has “very high energy storage density, both by weight (8.7 kWh/kg) and even more by volume (23.5 MWh/m^3)”.
If you can avoid transportation, that’s a 70% efficient means of storing energy indefinitely with current technology.
https://www.hydro.com/en/global/about-hydro/stories-by-hydro/the-worlds-most-energy-efficient-aluminium-production-technology/
https://doi.org/10.1016/j.ecmx.2019.100017
Wow… Where to even start…
Those numbers are damning. Being able to recover “up to 90%” of the Gallium/Indium in a laboratory makes it wildly inefficient. Even 99%would make this useless. If it was 99.99%it might be useful.
As energy storage, it is comically inefficient. The electrical energy and petroleum fuel cost of refining that Aluminum is an order of magnitude more than you could get out of the resulting Hydrogen.
Talking about this like it is some magic portable fuel generator is absolute nonsense. It “only” requires you to carry around a highly reactive metal that wants very VERY badly to oxidize. This isn’t a bar of Al. This reaction requires huge surface areas to be useful, and the metal will happily oxidize in the air before you use it. Oh and some other expensive metals. And then you need to carry the spent metals around too, which will be more massive due to being mixed with everything that isn’t Hydrogen from the sea water.
This very idea, as it currently exists, is laughably bad. Hydrogen is a tough thing to store. But this looks worse than just electrolysis in every single way.
In this application, you wouldn’t need to store the hydrogen, instead burning it on production. Your “storage” would be the very water your floating on.
Not that I don’t believe you, but citations please. You complain about the numbers but don’t offer any of your own. How much energy is consumed in Al production compared to when it’s oxidized? How much do the rare earths that are lost cost as a percentage of that?
clearly not by reading the paper
Not a bar, Beads of aluminum that have been treated with 5-8% gallium/indium alloy to create an infiltrated surface coating that allows the water to penetrate through the oxide layer to react directly with the aluminum bulk
90 tp 100% recovery
Recycled SEVERAL times.
As for the electrical energy and petroleum fuel costs of refining aluminum, Electrolytic deoxidation in molten salt (FFC Cambridge) could significantly reduce that overhead.
Thanks for this sniff test.
Does not pass.
Yeah, yet another “wow, metals and water make hydrogen!” boondoggle. Anyone who took high school chemistry knows that already. It’s not practical as either a source or a store of energy. Even hydrogen itself is impractical for most uses other than as a lifting gas, which ironically is banned for commercial purposes. I really just wish that scientists would stop doing this nonsense cash-grab type of research and that journalists would have the integrity to not hype it up. But alas, it’s 2024, and everything on earth is clickbait.
All available powdered aluminum supplies are needed for teenager ‘science experiments’ anyhow.
Drink more beer, recycle more cans, generate more hydrogen…. it’s a win win….burp…..
Party on Garth!
“High school Eagle Scout manufactures nanothermite. Family homeless.”
Not saying it never happens.
But I survived to adulthood.
IIRC We only stated on fire in a house, and were able to put that out with no adults finding out. Back when we were just discovering empty whiskey bottles could be warmed and made into onetime use fire throwers. About 14 years old. I still can’t stand the taste of Jack Daniels. Lessons were learned.
2Al(s)+6NaOH(aq)+6H2O(l)→2NaAl(OH)4+3H2(g)
All the hydrogen you could ever want (often much too quickly), from an entirely electrically reversible reaction.
I have no clue what they’re trying to achieve with this ‘research’.
2Al(s)+6NaOH(aq)+6H2O(l)→2NaAl(OH)4+3H2(g)
Lost something in the cut and paste. Please don’t try this without adequate comprehension. It’s spectacularly exothermic.
I give up. Look it up for yourself.
Be warned, this reaction exothermic to the degree that even trying to write the formula will fail when no precautions are taken
In words, solid aluminum rapidly reacts in a solution of lye to form sodium aluminate, hydrogen, and a bunch of heat. But I don’t know anything about reversing it electrolytically at that step. As far as I know, it’s taken to the hydroxide, calcined to get the oxide, and then fed into hall-heroult just like what came from mined aluminum. That hall-heroult electrolysis of the dissolved alumina is still very energy intensive and still consumes the carbon anodes even though you’re reusing the aluminum atoms instead of mining more.
“I have no clue what they’re trying to achieve with this ‘research’.”
More research MONEY. (looks like a hype and/or scam)
You can use stainless steel shaving currie qs almost like a bunch of fuccelli pasta looking with a massive myriad of surfaces that a salted or or a water with a citrulene or just lemon liens catalyst can with a mild electrical current create a huge amount of hydrogen or oxygen bubbling off which ever polarity it is situated in the water calories and the two games rise to the surface and can can be siffoned or pulled away into containment or directly fed into engines or fuel cell engines..
The background section of EP1301433A1 lists some of the patents field, going back to 1909. While a rather useless patent in itself, it seems to be a good entry point for patent research on the topic.
https://patents.google.com/patent/EP1301433A1/en
Al is much better for heating than applications aiming solely at producing H2 or electricity.
While on the subject of heat generation, it gets a lot worse (in terms of transportation) with iron powder:
https://innovationorigins.com/en/500-households-are-warm-thanks-to-rechargeable-iron-powder/
(and guess what, you need H2 to reduce the iron powder again…)
Perpetuum mobile. Use energy to produce pure aluminum, use pure aluminum to generate less energy than was used to produce the pure aluminum . . .
Nobody referred to this as being a perpetuum mobile, the aluminum is the fuel, fuel that needs to be processed to the proper state in order to be converted in the desired elements for the desired method of extraction of energy. In essence this isn’t much different than any other fuel that needs to be processed before it can be used. We can’t burn trees directly, we need to chop it down, dry it and cut it in into usable pieces. Gasoline comes from heavily processed oil. Although lot’s of work needs to be done, this is a very interesting concept.
It’s actually quite common to find wood laying on the ground and just burn it but the proof requires partial differential equations and is beyond the scope of this post. Gasoline is like falconry: for women and children! Real men hunt with eagles: kerosene.
Natural gas while requiring less work still benefits from compression. Atmospheric pressure is only your friend in one respect.
Natural wood burning on the ground reminds me of the first time I ever had a wood stove. And I often tell the same to people who think about going down that path.
My first year with a wood stove it was like something from heaven. All of the wood debitres from the trees around the house and all of the little pieces of scrap from the shop etc. All of it got burnt. It was wonderful. Did not even had any kind of a saw.
The second year, the radius had to expand a bit and I got a bow saw and an axe. There were still a lot of bigger hunks of stuff the wind brought down around the place but it was a bit more work bringing it in.
The next year, had to get a chain saw and a wheelbarrow and extend on into the woods, looking for downed stuff. It was starting to turn from a hobby into a job.
The next year it was looking for dead trees or trees that were choking out other trees or trees we did not like. Got a trailer to pull the stuff back with.
I saw how this was going and I could look on line for folks with the please come and clean this downed tree mess up for the wood things but I also had a full time job, and wood started to look just like any other fuel only it sucked. When I say that I mean you get up in the AM and stoke up the woodstove, you can cook over it but by the time it is doing any heating you are gone for the day. And when you come back, you stoke it up for dinner and the house is warm by the time you go to sleep, and cold again by the time you get up. Starts to make gas where you just set the thermostat seem pretty damn efficient.
One of my buddies just refuses to give on on calling it free. He spends almost every free moment he has going out to fetch down trees in people yards. He has two tractors, two trailers, 4 chainsaws, one splitter, and a host of other peripheral stuff all to have the privilege of burning free wood.
This seems like it takes a whole lot more energy to make than you get. I think the future of the world really depends on the sun, but not so much with solar cells but with giant 60 foot high evaporation engines aka drinking birds with giant magants and induction coils on them. Of course we would need a new 2Hz AC standard and have to deal with china for a continuous supply of butt feathers and top hats for them.
Your saviour is a multifuel stove.
You collect free wood at a low rate during the year.
But alongside the wood you can burn coal, plastics, paper, you name it.
I am at level 2 in your story, and have been for 20 years.
It returns less energy than was invested, rather than more as fossil fuels do. Whereas, once you have a battery you can recharge it many times and most of the energy you spend will come back out. So to replace fossil fuels, we need to address both their utility as a storage method as well as their addition of energy to the system. Making a fuel artificially from renewables fails in keeping up with the latter, and that limits the conditions where it’s good.
It seems like they are on track to rediscover cathodic protection, 200 yrs later.
It seems that many of commenters here have not read the whole artice or should maybe read it one more time. The whole idea here is to use ready made aluminum add gallium and other metalls and coffe ingridients to make hydrogen on demand as. This could be very useful on ships etc. The hydrogen is then burned in engines og used in fuel cells. That way a large ship could be tanked with aluminium in raw form like ingots or whatewer and be mixed to a alloy at room temperature onboard for this process. Genius
Genius?
I guess that words means less than ‘extreme right wing’ now.
Aluminum production: 30-40% efficiency in converting aluminum oxide to aluminum via electrolysis
Hydrogen generation: 50-60% efficiency in producing hydrogen from aluminum
Fuel cell conversion: 50-60% efficiency in converting hydrogen to electricity, or 25-30% efficiency if using hydrogen in an ICE.
The overall round-trip energy efficiency for this process is about ~7-15%, if using fuel cell and electric motors, or ~3.5-8% for an ICE. It’s very low efficiency, but I guess it’s potentially useful as an energy storage mechanism when midday solar causes electricity prices to go negative.
The best part of waking up is Folgers making hydrogen.
Maxwell House….good to the last hydrogen
It all starts with Nescafe and aluminum
It’s not just coffee… it’s Starbucks and aluminum making hydrogen
Keurig….brew….enjoy…. hydrogen
Well you can decompose h202 and get free oxygen and hydrogen
And enough to probably run a car engine
Probably way more effective than hho alone
Waste heat used to roast the coffee beans!
Read a paper on naturedotcom that warned of the adverse effects of hydrogen bonding with methane in the upper atmosphere.
Just use sodium instead. Don’t need any of these fancy catalysts to make it liberate hydrogen. Bonus: BY VOLUME, it’s the cheapest metal you can buy. /s
“Aluminum holds twice as much energy as diesel, and 40x that of Li-Ion batteries. So finding a way to harness that energy could have a huge impact on the amount of fossil fuels burned by humans!”
You mean the energy that was put into the raw ore to get pure aluminum? Minus some efficiency factor… Much like hydrogen, aluminum may be an energy storage solution but not a generation one.
What kind of byproducts does it release at industrial scale into that seawater and what do those things do down the line. You’d think that the renewable energy people would be used to answering this question by now
Great idea. But Gallium and Indium are in relatively short supply as it is, and mostly originate in China that is well known for its “environmentally conscious” mining methods.
Even with a recovery rate of 90% the economics are not there from that one aspect alone. So the idea needs some more work.
I find myself wondering if bauxite could be used in a “tower of power” solar farm, because if so there’s your hot aluminium, gallium, and electricity supply right there.
If you are using it on a cargo ship, why not just chuck the Al powder into the engine?
Those big diesel engines aren’t fussy.
Page 12 of the report states:
“after letting it react in DI water for 24 h, the eGaIn separated from the other material, and re-weighing it showed recovery ratios ranging from 90% to 100%”
Meaning the precious metals used in the aluminum have the potential to be recovered in its entirety when done right.
If it can be refined into a package that maintains the 100% recovery ratio this could very well be the holy grail of hydrogen generation.
KOH water and aluminum seem to work really well in releasing hydrogen. Why waste time with expensive catalysts?