Water is an excellent coolant, but the flip side is that it is also an excellent solvent. This, in short, is why any water cooling loop is also a prime candidate for an interesting introduction to the galvanic metal series, resulting in severe corrosion that commences immediately. In a recent video by [der8aer], this issue is demonstrated using a GPU cold plate. The part is made out of nickel-plated copper and features many small channels to increase surface area with the coolant.
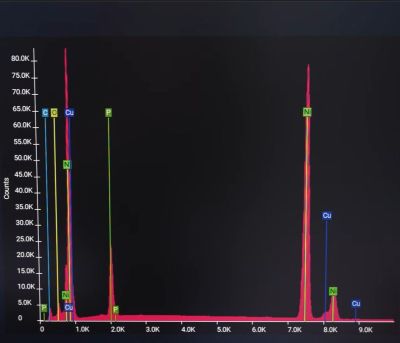
Theoretically, if one were to use distilled water in a coolant loop that contains a single type of metal (like copper), there would be no issue. As [der8auer] points out, fittings, radiators, and the cooling block are nearly always made of various metals and alloys like brass, for example. This thus creates the setup for galvanic corrosion, whereby one metal acts as the anode and the other as a cathode. While this is desirable in batteries, for a cooling loop, this means that the water strips metal ions off the anode and deposits them on the cathode metal.
The nickel-plated cold plate should be immune to this if the plating were perfect. However, as demonstrated in the video, even a brief exposure to distilled water at 60°C induced strong galvanic corrosion. Analysis in an SEM showed that the imperfect nickel plating allowed copper ions to be dissolved into the water before being deposited on top of the nickel (cathode). In a comparison with another sample that had a coolant with corrosion inhibitor (DP Ultra) used, no such corrosion was observed, even after much longer exposure.
This DP Ultra coolant is mostly distilled water but has glycol added. The glycol improves the pH and coats surfaces to prevent galvanic corrosion. The other element is benzotriazole, which provides similar benefits. Of course, each corrosion inhibitor targets a specific environment, and there is also the issue with organic films forming, which may require biocides to be added. As usual, water cooling has more subtlety than you’d expect.














can’t you just drop a block of zinc in the reservoir or something?
PC water cooling is a bigger pain in the back side than most people realize.
Go to the goodie store buy an AIO and throw it away when it starts making gurgling noises.
Putting sacrificial metals in the system only gums it up. Those ions all have to live somewhere……..
The only Good Alternatives (long chain fluorocarbons like FC-40, 70 or FC-35.
We are Stuck with Water……
Life sucks sometimes…….
Scrap what you have and go with mercury. Just avoid aluminum components.
Where is your circuit? Sacraficial annode would need to connect to something. If it’s just floating free it has no meaningful effect.
This is a chemistry issue not an electricity issue.
Sorry, but the word “anode” has meaning here. These have to be electrically bonded into the system that is being protected or the cathodic metal won’t be protected. Measured voltage potential from boat zincs is only a fraction of a volt but I’ve always wondered if you couldn’t power something with that.
the people installing the zinc anodes would probably thank you not to turn it into a battery and accelerate the corrosion.
where’s the circuit in the water coolers that are already experiencing galvanic corrosion? isn’t it the fluid? even if they somehow manage to use sufficiently well distilled water and avoid introducing ions while filling the loop, and keep it form absorbing anything out of the air (which is pretty unlikely), if there’s any galvanic corrosion occurring, then the instant it starts there will be enough ions in the water to make it conductive and then you’ve got your circuit.
So if you put in the anode without connecting it then you basically are getting both to corroded without affecting each other. Once you connect them you get the electronegativity potential from one added to the less electronegative element basically zinc wants to corroded more so it will eat through the zinc first when they are connected. Otherwise they will both corrode at their own rates, not one after the other.
Why not use modern motorcycle coolant? LIQUI MOLY Radiator Antifreeze Universal Radiator Protection Item No.: 21313. This is safe for copper, aluminum, etc.
DowFrost HD works. I’ve used it for 5+ years in a loop made of cheap aluminium blocks with cheap mixed metal fittings.
Think about all the different metals and alloys in an automotive coolant loop. Copper, Zinc, Iron, Steel, Nickel, etc.
All those same metals occur in other heat exchangers, and nobody has serious issues with galvanic corrosion as long as they add a glycol anti-freeze with corrosion inhibitors.
The main issue with the glycols in anti-freeze additives is that glycols attack most of the cheap and “pretty” tubing materials in PC water cooling loops.
The issues still exist, just are minor enough that they are factored into design headroom. Home computer coolants are at much lower pressures and features are much smaller. e.g. you can use the same equipment, if the water block is designed for it, at lower efficiency.
Really, DIY systems should have no issue beating out AIOs in this respect using even only water, and larger radiators are readily available As you have noted.
I suspect that part of the issue is just that PC water cooling exists in sort of a weird niche that puts a lot of pressure on it, not all in the direction of expertise.
As with any hobby; some people involved are really serious and really good; but it is one where things you previously could only DIY with water cooling(back when ‘CPU heatsink’ meant some random aluminum extrusion that shipped with a 30ish watt CPU; because what more would you need?); because improvising heatpipes is a real challenge and getting nontrivial metalworking with nothing but a few basic hand tools is fairly arduous are now largely professionalized.
Air cooling’s ‘baseline’ for vaguely serious performance enthusiasts is now the bunch of direct contact heatpipes with a 120 or 140mm fin stack, available for a relative pittance; works great.
Liquid AIOs have gone from being basically exotic scams to being still more expensive than air cooling; but increasingly cheap and reliable: get 120mm at some distance from the socket or 240/360mm just plug and go; shouldn’t need to worry about the working fluid for 5 years, fine.
That leaves the people doing custom liquid loops in a fairly narrow niche, mostly for its own sake or to satisfy particularly specialized aesthetic or performance requirements; and often bombarded with a unhelpful mix of painfully expensive parts from the reputable outfits; or sometimes-great-value-sometimes-total-scam options from the oddballs who could be the same thing that the brand you’ve heard of is rebadging and quadrupling the price of; or could be outright lying about even basics of composition and don’t even think about the workmanship.
It’s not like professionalism is dead; but custom loops are a very constrained market when even ‘gamer’/’workstation’/’enthusiast’ thermal scenarios are typically addressed by heatpipes or AIOs; and all the real money in liquid cooling is for high density datacenter compute products; so you’ve got a mixture of professionals charging the prices you’d expect for a niche product, competent amateurs; and optimistic kids trying to get the look they want based on internet forum rumors and pinching pennies on fittings from vendors who may or may not even commit to what metal they are made of; much less bother lying about it.
Ten years ago we were operating a supercomputer in which the cooling circuit contained several different metals. After 5 years of operation, some cooling radiators were clogged with sludge due to galvanic corrosion. That lead to cooling problems, and it was a headache to clean all the stuff.
A colleague of mine, who had some knowledge of chemistry, explained to me that this was a really bad idea / misconception. I think that this cooling system was planned to be used for less than 5 years (some kind of trade off between price versus durability).
At the same time I experienced this problem when I built my microbrewery: I had the bad idea of using different metals for my brewing kettle: aluminum (the kettle) and stainless steel (the stirrer). After a 10 minuted test to clean the kettle (with water and dish soap), a dark gray juice came out, and my aluminum kettle lost 1/10 of a millimeter. There were electrical arcs between the kettle and the support that held the stainless steel mash. I rebuilt it using only stainless steel, and now it’s fine (so the beer is, too ;).
So I’ve learned that rule: never mix metals in a wet design unless you want to make a battery.
In most western countries (and all of the EU) you are not allowed to have aluminium pots and pans and kettles; because the aluminium gets into your brain and does bad things.
And of course they use a separation layer in aluminium cans to avoid direct contact.
Oddly enough though everybody uses aluminium foil all over the place for cooking, including commercial places that sell food.
Humans eh, no wonder AI gets confused..
Anyway, perhaps you should not use aluminium for your brewing that reason too.
I live in the EU and own an aluminium kettle and an aluminium cooking pot with only the normal level of anodising, I do not fear a knock on the door from the gendarmes. They are not illegal. They are on sale freely. I agree that stainless steel, cast iron, tinned copper etc are better materials in general.
In Sweden, no aluminium stuff.
Amazingly old news. Military application engineers figured this out way more than a century ago. Use DI water, add a “water wetting agent” if desired, and anodes. Change anodes as they suffer galvanic corrosion. Periodically, power down, drain, and clean the heat exchangers. Rinse, repeat. This is literally not rocket science; it is middle school chemistry. And anybody who has ever worked in a professional capacity on heat exchangers SHOULD be aware of this. We do it on cars, airplanes, earth moving equipment, naval vessels, and power plants.
The single piece of cookware I own that has “proudly made in Sweden since 1925” on it, is decidedly made from alumin(i)um. Trangia branded cookware for hikers. Bought recently. Still for sale. Bloody expensive, too.
My kettle is a TRANGIA ( Swedish). Amazon.fr also has ordinary kitchen kettles in aluminium though from a brief look it seems that aluminium cooking pots and pans are coated in some sort of enamel to keep the food away from the aluminium.
I still think that you can own and use bare aluminium pots and pans in the EU, if you want to.
Are you sure? It is very peculiar, they were removed from stores ages ago (decades?) and even from discount stores, seems very odd that they would be available in France or Belgium (based on your use of ‘gendarmes’). Now in eastern Europe I can see it slipping through the cracks, but more western EU, that’s very peculiar.
I mean yes internally lots of kettles and pots and pans are aluminium, but there is always a barrier layer like anti-stick coating or stainless or some such surely?
Anyway, I would avoid it since you can easily do so.
“In most western countries (and all of the EU) you are not allowed to have aluminium pots and pans and kettles“.
Trangia (Swedish) use hard anodising now so I suppose they just scrape through the new regulations. What I was trying to say was that I very much think that the Gendarmes will not arrest me for owning and occasionally using aluminium cookware. (My Trangia cookware is decades old).
I tried to fact check this conspiracy theorist thinking. At least the US and UK versions of the Alzheimer society say there is not convincing link between metal ions and that particular disease. Depending, of course, on your definition of “does bad things.” And those are non-profits seeking to help with that disease so I don’t think they would have ulterior motives. that aside, as noted below, other materials are generally superior but more expensive. #tradeoffs
common deodorant has aluminum in it, and we get more aluminum from rubbing deodorant under arm than we get from cooking food in aluminum pots.
I am still using 70+ years old Club pots and pans, it heats well and is easy to clean. No teflon to flake off and cause problem (some of the very late produced Club did have teflon before Mirro bought them out)
“In most western countries (and all of the EU) you are not allowed to have aluminium pots and pans and kettles; because the aluminium gets into your brain and does bad things.”
No. This is false, though there are requirements about anodization to minimize leaching. As to health hazards:
https://wwwn.cdc.gov/TSP/ToxFAQs/ToxFAQsDetails.aspx?faqid=190&toxid=34
Yeah uhm thanks but the CDC is US and the EU and US don’t quite agree on everything.
And of course money talks even louder in the US than the EU… over shouting health concerns possibly.
And why risk it if you don’t need to? Because the US says so? I think that’s not a good measure of things.
But you are of course free to disagree and to use it as you wish, and it’s not a bad thing to share the official US views on this so thanks for that. Better to share the CDC site than shout some random unsubstantiated personal view or something some vlogger shouted :)
My mother used to make applesauce in an aluminimum pan (40 years ago) and I never liked it much because it had a weird taste. At some time she started making it in a stainless steel pan and then it tasted much better. The difference was really obvious to me, but apparently not so obvious to other people in my family. The insides of her old pans were also not smooth anymore (like the outside). They had clear signs of pitting corrosion.
I’ll do some speculation here; perhaps an expert can correct my mistakes. Aluminum exposed to the air forms a fairly strong oxide skin, which will protect the aluminum from corrosion except from particularly strong chemicals, high heat, or severe mechanical abrasion. If your mom cleaned the aluminum pan with steel wool (a Brillo pad) that would have removed the oxide layer. Dishcloths and some of the modern plastic scrubbers would leave the oxide intact.
Aluminum alloys change the risk. On the one hand, the alloys can be stronger and more corrosion resistant. On the other hand, galvanic action between different metals in the alloy is a potential problem, particularly if the alloy is not uniform.
This is really good, and nails most of what goes on while canning.
However, there’s often heat and acid working at the same time, to cook your jam/applesauce or whatever, and the acid is a preservative, to keep the pH low enough that your food stays fresh/doesn’t spoil while in storage.
So I think the acid in the aluminum pot can strip enough of the aluminum oxide to expose bare metal, which can get into the applesauce as dissolved ions.
Leaving strong acidic food like tomato sauce for several hours will cause high aluminum leeching. When done cooking, remove them quickly then rinse out cooled pots and wash them with non-abrasive pad or cloth.
…i wish Ford had respected that notion when they saddled millions of late 1970s cars with their “Variable Venturi” carburetors, which would routinely load the float bowls with grey sludge, from the decomposition of the aluminum parts used in “wet” internal areas… at the time I was stuck learning this (mid 1990s) by way of failed smog checks, it was widely considered unrepairable (you couldn’t have added or changed anything to prevent the galvanic activity without drawing an equipment violation), unreplaceable except with same P.O.S., or finally in the mid 1990s with a mid-priced Holley…
A LOT of solid 302 cid powerplants met early graves due to their fuel systems being factory-sabotaged that way…🤦♂️
(Addendum) the mechanics KNEW itnwas galvanic decomposition, you justnwerentnallowed to solve the problem under our ridiculously misguided smog laws of the day.
Hi! Are you a bot?
What if we used monel for water cooling setup? Typically contains 66% nickel and 31.5% copper, with trace amounts of other elements like iron, manganese, carbon, and silicon. It has been used in marine for around 100 years, and where corrosive chemical have been used. Seems like it’d help resist corroding in water block and radiator. Nickle may not be as good a conductor of heat but it should make waterblock last a lot longer. Useful if your water setup has a removable and replaceable water pump
I would be willing to bet it is not a very good thermal conductor.
I have always wondered why the interface surface between the Waterblock and the CPU IHS were not plated with Gold, number Three when it comes to thermal conductors.
Diamond… (The Very Best)
Silver… (cant use this its too reactive)
Gold… Why not????
Only $3,400.00 per OZ this morning.
Industrial diamond is a ‘cheap enough’ material.
Less than gold.
Don’t think gold will wet diamond powders though.
Your chasing decimals.
IIRC an equal weight of aluminum is a better heat sink than copper, despite being worse thermal conductor. For most definitions of ‘better’.
Thinner water block walls of stronger/more stable material would give similar results.
Quote Warner Von Braun: ‘Tell the bean counter we’re using a gold mirror because solid gold would be too heavy.’
The mirrors on the James Webb Space Telescope are coated with gold, because it reflects 99% of infrared (basically anything above 600 nm). So even though gold is good at conducting electricity (2.44 x 10⁻⁸ ohm/m resistance), and thermal conduction ( 315 W/m.K), any radiated heat will be reflected back to the source extremely efficiently.
I am still a fan of plain o’ air cooling. None of my machines has ever been pushed enough to need liquid cooling from the Z-80 to current processors. I am not an over clocker. I even turn off the ‘boost’ mode in BIOS and run at the given CPU recommended GHz. And the machines still feel super fast for what I do. In today’s world, I don’t think we really have a need to ‘push’ the bleeding edge for home computing tasks. As with anything there are edge cases…. But in general… At least that is my opinion!
Thanks for the article. One of things I felt could be a problem with liquid cooling over the years, turns out to actually be a ‘real’ problem.
We use stainless steel and cast iron pans around here. We used to use Aluminum, but moved away from them. Not the problem they are made out to be though. Just like lead isn’t either. But we’ll always have the sky is falling, rooms with round corners, and dull knife crowd with us forever.
I like positive pressure air cooling with good intake filtration for my computers. It’s simple, cheap, lightweight, and maintenance consists of little more than cleaning the intake filters occasionally. Positive pressure means dust doesn’t get sucked in anywhere else. With the right choice of CPU heatsink and fan there can be plenty of cooling capacity for boost modes and even enough for moderate overclocking.
We used to have fully immersed mineral oil PCs until some stupid patents ruined it.
What if we changed the loop to use mineral oil instead?
Crazy over clockers have a nonconductive liquid that they like to cool with liquid nitrogen (till slushy), then recirculate. Expensive and pointless, but whole machine immersed.
Haven’t even looked at their shenanigans in decades tough.
IIRC someone had a 1 gig Pentium 3 running at something like 6 gig.
Those guys are into getting screen caps of super high clocks.
Machines are barely stable enough to boot and run a benchmark.
Makes ME want to hang them from doorknobs by their underwear.
Compared to water, mineral oil is about a third as effective at heat transfer and also an order of magnitude more viscous (at startup/room temperature anyway).
A standard water cooling system swapped over to mineral oil would suddenly become extremely underpowered and undersized, assuming it worked at all.
Of course you could build a (very) custom loop tailored to mineral oil, but at that point it’s probably easier to just do immersion cooling (which is heavy and messy, but allows you to just use your existing heat sinks rather building huge, custom, chunky “oil blocks” for your CPU/GPU/etc)
Could you stick de-ionizing filter chambers in the loop to keep the dissolved metals down?
Deionizing filter? Like an RO filter? The power requirements to pump through that at typical flow rates would be pretty damn high.
All that heat eventually needs to go to the air anyway, unless they’re running the system off the tap and into the drain, and then what’s the fuss about corrosion?- these guys think they’re cooling a nuclear reactor, and the gimmick factory knows it. There is NOTHING wrong with air cooling (esp. forced, try a CPAP blower for noise reduction) for individual computers and components. Try it sometime.
makes me wonder about my hydronic system, which is i think sheet-steel radiators, pex pipe, and brass fittings.
As long as the metal parts are insulated from each other (e.g., not grounded) then no current will flow, so at least the galvanic corrosion won’t be an issue.
yeah i was kind of hoping that the ten feet of pex (insulator) between each part would help me out but then i remembered each radiator has a direct brass-on-steel coupler between it and the pex. if i ever have to maintain one of those, i’ll be curious what it looks like when i take it apart..
i hope that since reading plumbing faqs didn’t mention it, the margins must be fine. they did warn me about running acidic condensate drain through brass and hoo boy that didn’t take long to see in action (i only did it because the part was on hand, i knew i would eventually have to replace it with plastic).
Didn’t this get solved by the automotive industry decades ago?
Deionised water mixed with glycol and corrosion inhibitors.
Works fine with cast iron, aluminium, and steel all in the same system.
Slightly less effective than water alone, but it’ll last years without issue
Just buy good stuff and don’t settle for crap disguised as high end thanks to marketing campaigns (looking at you, EK).
Aquacomputer’s DP Ultra has been in my Aquacomputer/Watercool loop since I built it three years ago and the conductivity hasn’t changed since the initial air purge. Was at 11 microsiemens per centimeter for the first week and has stayed at 8 microsiemens per centimeter and 100% quality (none of the anticorrosion or antimicrobial agents have come out of suspension) since. There’s no staining or leeching on any of the blocks, all of the nickel plating is intact, and there’s no fog on any of the acrylic. Loop contains three all-copper radiators, two nickel plated copper GPU blocks, one nickel plated copper CPU block and is perpetually pressurized to 420mbar thanks to the vacuum pump. They recommend changing the coolant every year or so, but I haven’t felt the need to.
You get what you pay for, so… buy once, cry once.