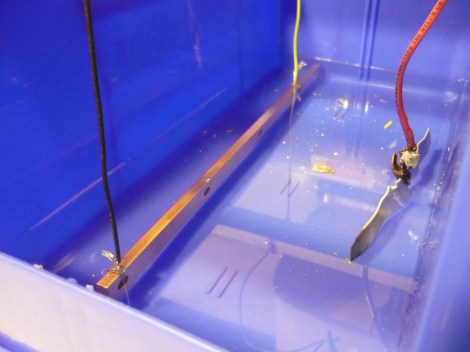
Copper bus bars are commonly used instead of wire for carrying high currents. [Dane] needed some bus bars for a project, but he was worried about corrosion. His solution was tin electroplating the bus bars to lower the risk of corrosion while keeping the conductivity high.
The process requires only two chemicals: hydrochloric acid and tin. The electrolyte solution is made by dissolving tin into the acid. Then the bus bar is placed in a diluted solution and a 1 A current is run through it. The result is a fine coating of tin on the copper, which will not corrode in water.
[Dane] mentions that he’d like to try the process with silver solder in the future, since it is easier to find than tin. He also wants to find a way to measure the amount of tin deposited onto the bus bars. This process could be helpful for anyone who needs some corrosion resistant high current conductors.
Check out a video of the plating process after the break.