The 1970’s was the decade that illuminated the threat of acid rain to the citizens of the US. It had been known to exist several years before, but the sources of the problem did their best to suppress the information. It wasn’t until the environmental damage became significant enough to draw national attention that it would lead to the US enacting regulations to stop acid rain.
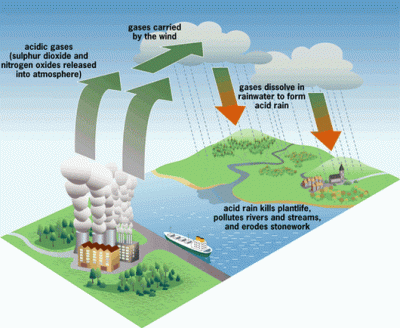
Truthfully, most of the public was probably still unaware of what acid rain actually was. The default mental image that comes to the mind of the non-chemist is large drops of battery acid raining down from the heavens and devouring everything. This is not quite the case, however. Pure water has a neutral pH of 7. Normal rain is actually slightly acidic as it picks up CO2 from the air, making carbonic acid. But when this “normal” rain mixes with the byproducts of industrial plants that pump out large amounts of SO2 (sulfuric dioxide) and NO (nitrogen oxide) into the atmosphere, it becomes even more acidic – down to a pH of 3. This “acid” rain has the acidity of citrus juice, so it’s not going to set the world on fire. But it will wreak havoc on local ecosystems.
The 1990’s brought with it tough government regulations on the output of SO2 and NO by large factories, pretty much eliminating acid rain in the US. The rise and fall of acid rain is a great example of why we should educate ourselves on the basic chemistries that define our lives, even though we might not be actual chemists. In this article, we’re going back to your first year of college and hash out just what defines an acid and base. And solidify our understanding of the pH scale. It is essential for the future biohacker to have this knowledge in their toolbox.
The Power of Hydrogen
Our journey begins with plain old water. You probably believe that pure water is nothing but H2O. The problem with this picture is that water has a tendency to auto-ionize. What happens is that a hydrogen atom will break off of one water molecule and join another. This results in two molecules – H3O (hydronium) and OH (hydroxide). As you might have already guessed, the +H3O has a positive charge and the -OH has a negative charge. The process forms an equilibrium in water at 25 C, so that solution remains neutral. This means that pure water at 25 C is composed of H2O and equal parts of +H3O and -OH.
Adding different molecules to the water can disrupt the balance of the hydronium and hydroxide ions.
- When [+H3O] = [-HO], the solution is neutral.
- When [+H3O] > [-HO], the solution is an acid.
- When [+H3O] < [-HO], the solution is a base.
The number of +H30 ions in the solution will determine how acidic it is. Generally, chemists use the mole to count the number of atoms or molecules in a sample. The mole is kind of a “chemist’s dozen”. A dozen is a 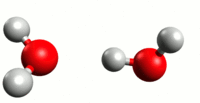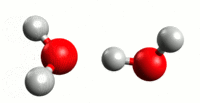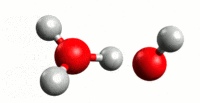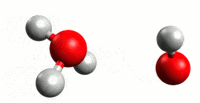 count of twelve, a couple is the count of two, and the mole is the count of 6.022 x 1023. However, the number of +H3O ions present in an aqueous solution can vary by several orders of magnitude, so another type of scale was created to better quantify this amount. It’s called the pH scale, with pH standing for “power of Hydrogen”.
count of twelve, a couple is the count of two, and the mole is the count of 6.022 x 1023. However, the number of +H3O ions present in an aqueous solution can vary by several orders of magnitude, so another type of scale was created to better quantify this amount. It’s called the pH scale, with pH standing for “power of Hydrogen”.
To avoid getting our brains tied up in not-very-helpful conversions, take it as a given that the concentration of +H30 ions in neutral water is about 1.0 x 10-7 molar. So we say this neutral water has a pH of 7. As the number of +H3O ions increases, the pH decreases. Orange juice has a pH of about 3.5, which means its +H3O concentration is 1.0 x 10-3.5 M. Ammonia has a concentration of 1.0 x 10-11 M, which gives it a pH of 11. You get the idea. Simple, isn’t it!
The pH scale is logarithmic, so acid rain with a pH of 3 has 10 x 10 x 10 or 1,000 more times the amount of +H30 ion concentration that typical rain water, which has a pH of 6.
Johannas Bronsted
In our previous article, we defined an acid as a substance that produces hydrogen ions when dissolved in water, which is known as the Arrhenius model. While this definition is sufficient for most of what you’ll run across as a biohacker, we should take the time to mention a more inclusive definition developed by Danish chemist Johannas Bronsted in 1932. His definition can explain acids and bases without the need for them to be dissolved in an aqueous solution. But it’s a little more in-depth.
Consider the above example of how water will autoionize. We explain this by a hydrogen atom breaking free of a water molecule and then joining another to make H3O, and leaving an OH in its wake. While this is helpful for explanation, it’s not entirely accurate. When the H atom leaves its water molecule, it leaves its electron behind. And since a hydrogen atom consists of one proton and one electron (there is no neutron in H), that means it’s basically a free proton. Now, this proton has a positive charge, and the oxygen in water is slightly negative. So the proton will be attracted to the oxygen atom on other H2O molecules. H3O could be written more accurately as [H2O * H+]. With the understanding that free hydrogen ions in an acidic solution are (hydrogen) protons, a better definition can be developed. The Bronsted definition defines an acid as a solution with proton donors, and a base as a solution with proton acceptors.
Consider the example of hydrofluoric acid. When you mix hydrogen fluoride (HF) with water, the hydrogen atoms and fluorine atoms break apart is a process known as dissociation. The hydrogen atom leaves its electron with the fluorine, so you wind up with something like:
HF(aq) + H2O(l) <–> +H3O(aq) + -F(aq) (aq) means aqueous and (l) means liquid state of matter
By the Arrhenous definition, the HF makes free hydrogen ions and is therefore an acid. With the Bronsted definition, the HF donates a proton (the hydrogen atom without its electron), and is therefore a proton donor. Which also makes it an acid.
You should now have a basic idea of what an acid and base are, and why they are defined the way they are. This essential knowledge is needed before we take on the much more exciting amino acids – the building blocks of proteins, and all life on earth.















It cannot be over emphasized that pH is a log scale.
pH 7 = neutral
pH 6 = slightly acidic
pH 3 = orange juice
pH 1 = stomach acid
pH 0.8 = battery acid.
I’ll drink anything down to pH 2. I live with pH 1 in my stomach but I have painful acid reflux capable of dissolving tooth enamel and promoting esophageal cancer.
So that “small jump” from 1 to 0.8 is the difference between business as usual and sudden, painful death.
Technically, stomach acid is really friggin strong. Your stomach deals with it by continuously excreting thick mucus so it doesn’t actually touch the walls.
That’s why when you get a stomach ulcer, the bacteria cause a cavity in the stomach lining and the acid contacts your flesh directly, and that really really hurts.
I like to think of it in terms of decimal places.
PH7 = .0000001 concentration of hydronium (moves the decimal point 7 places to the left)
PH6 = .0000010 concentration of hydronium (moves the decimal point 6 places to the left)
PH5 = .0000100 …etc.
PH2 = .01
PH1 = .1
The intermediate values don’t match up (PH 1.5 is not .15), but it nicely shows the relation between the logarithmic values and concentrations.
As the FTA explains, learn your powers of 10 and all will be straight forward from then on. ;).
Same as had to do to stop the 0.00000…uF nonsense like the old 0.001uF for 1nF.
Defined PH := – log10([aH+])
so:
PH x = – log10(10-x) = x and that’s it!
No preview and <:sub> and <:sup>: don’t work in comments. :(
PH x = – log10(10^-x) = x and that’s it!
“As the number of +H3O ions increases, so does the pH” Should say “decreases” – and the neutral pH molality is missing a minus sign.
‘M’ means molarity, ‘m’ is used for molality though for clarity’s sake mol/kg is sometimes preferred, neither of which technically describe what’s happening in the pH scale. pH is the activity of H+, {H+} , which in many cases is effectively equal to molarity, but it comes up often enough to know this limitation
While aqueous solutions of hydrogen fluoride are certainly powerfully corrosive (and I have the scars to prove it) by definition it is a weak acid. The pKa of HF is 3.14, strong acids have pKa values of less than one. You would have been better off using HCl as an example, as it has a pKa around -7.
A lot of the challenges with aqueous HF are due to the F- ion itself (not just that it is an acid). Excess fluoride ion precipitates calcium (as insoluble CaF2) and so wreaks havoc on tissue, nerve function, etc. Aqueous HF will also etch glass due to the affinity of fluoride ion for silica.
“As the number of +H3O ions increases, so does the pH.”
You’ve got that backwards, increase of +H3O decreases pH
“neutral water is about 1.0 x 107 molar”
and missing a ‘-‘ sign there.
THANK YOU for finally embracing subscripts in chemical formulae!
Fixed, Thanks!
On the previous article, an error on the blackboard was pointed out. (Pedant) comment.
I am not commenting if the correction is correct. I am not a chemist.
The error was carried over to this update. Does only the users read the comments and not the authors?
It is correct, the left ‘CH’ should be a ‘CH2’ , carbon has to have 4 bonds on it in a molecule (neutral by definition) given the groups next to it it must be a CH2 and not a double bond.
*Right. apparently I don’t know which side is which.
Joe’s a phenomenal artist, but he’s not a chemist. And we’re not hassling him to re-draw the thing.
If you squint a little bit when you look at the banner, it’s just fine. Give that a try. :) Or scroll fast.
Right but pedant posted a corrected graphic. Go look, that one could have been used.
I am no chemist, but I did stay at a Holiday Inn.
Now have a huge capacitor with water acting at the plates and heat one side :D
Mole is not arbitrary measure – it is a factor which approximately gives us number of nucleons (protons and neutrons) in a gram of matter, so that chemists could use their legally calibrated weight scales to measure proportional amounts of molecules of pure chemical compounds, or number of atoms in an pile of a pure element. In imperial system, based on ounces, mole would have been a different number.
Obviously, the origin of SI system either pre-dates discovery of atomic mass quantization, or was rigged to make life easier for shipbuilders and other mechanical engineers, otherwise international system basic mass unit used in everyday life would had been chosen to be an exponent of 10, without that 6.022 mantissa, and then consequently, to appease shipbuilders, unit of length would had been chosen approximately as edge of an cube containing one thousand such mass units of water. How would that get harmonized with Earth’s meridian, I am too lazy to further contemplate …
If we ever get to the point to communicate with extraterrestrial intelligent life, we should probably define universal unit of length as wavelength of cosmic background microwave radiation.
Not all acid in rain is harmful, we get a big flush of vegetation growth from storms that consist of both a lot of rain and lightning. Interestingly the first storms of the season can be dry electrical storms that start fires which leave basic ash on the top of the soil ready to react with those acids. Nature’s own fertiliser factory.