There are a whole bunch of high school science experiments out there that are useful for teaching students the basics of biology, physics, and chemistry. One of the classics is the lemon battery. [iqless] decided to have a play with the idea, and whipped up a little something for his students.
The basic lemon battery is remarkably simple. Lemon juice provides the electrolyte, while copper and and zinc act as electrodes. This battery won’t have a hope of charging your Tesla, but you might get enough juice to light an LED or small bulb (pun intended).
[iqless] considered jamming electrodes directly into lemons to be rather unsophisticated. Instead, an electrolyte tray was 3D printed. The tray can be filled with lemon juice (either hand-squeezed or straight from a bottle) and the tray has fixtures to hold copper pennies and zinc-plated machine screws to act as the electrodes. The tray allows several cells to be constructed and connected in series or parallel, giving yet further teaching opportunities.
It’s a fun twist on a classroom staple, and we think there are great possibilities here for further experimentation with alternative electrolytes and electrode materials. We’d also love to see a grown-up version with a large cascade of cells in series for lemon-based high voltage experiments, but that might be too much to ask. There’s great scope for using modern maker techniques in classroom science – we’ve discussed variations on the egg drop before. Video after the break.
[Thanks to Tal Dayan for the tip!]

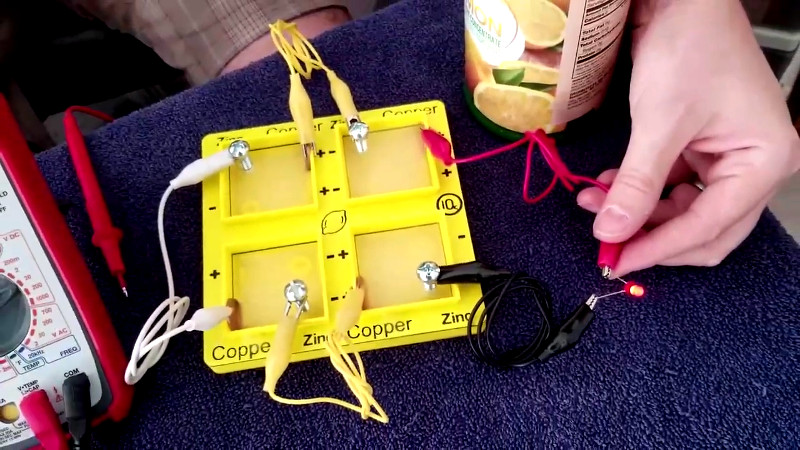














This is a great idea for school (but grade school, not high school), just one problem if he releases the build files some countries don’t have copper pennies, might need to tweak the design.
USA pennies are mostly zinc, they won’t work here. You’d need some rather old pennies to get copper for this. Household wiring are usually pure copper and should work instead of pennies. Hardware store has some by hundred feet or you could check construction site for short wire scraps (ask first!)
This is Patrick (iqless) modern pennies are not fully copper but they do have copper. I tried old pennies 1982 and before are mostly copper and newer pennies and both worked just fine :)
Iqless here, although 1982 and prior pennies are nearly fully copper and modern ones are not. I had no trouble using either to get the experiment to work.
EU “pennies” (cents) are copper clad iron – a good example of electrolytic corrosion, after the plating gets damaged and an electrolyte (moisture) comes to it. Basically a shorted-out battery.
But copper wire was never a problem to have, pure zinc was. But copper and iron also work.
I just wonder, why the cells in this nice experiment are configured for maximizing internal resistance: The electrodes in the corners to maximize distance and upright in a shallow pool of juice to minimize contact area. “How to build a battery” :-)
Really? So you believe that this article and the video are all some sort of hoax then? Why go to the trouble?
It’s one thing to listen to an UNTESTED idea and then tell a reason you think it will not work. It’s another to point at something that someone already built and did work and say “that won’t work”. You really believe your opinion is more valid than experimental results?
So.. here’s what else you missed. Galvanized screws aren’t mostly zinc either. They are mostly steel. So you have copper that’s mostly not copper and zinc that’s mostly not zinc you really think that will not work even after someone already confirmed that it does?
Consider this. The screws ARE mostly zinc in their outside surface. Likewise a modern penny IS mostly copper on it’s outside surface. Magnesium would not be that color. Who cares what is inside? The insides will never come into contact with the electrolyte anyway!
So remember… Observation beats pontification. If we could all just remember that then a lot of societies problems would just go away.
Non lemon electrolyte container and is that a bottle of pre-squeezed lemon juice he’s using? Lemon battery? Bah! I’d like to tell this where he can stick his electrodes.
A series of cells in an ice cube tray could be used for much higher voltage.
3D printing is not needed.
When life gives you lemons, don’t make lemonade, get mad!
Get your engineers to make a potato battery!
Do you know who I am?!
I’m the man whos gonna make a potato battery! With the lemons!
“Oh hi…. how are you holding up?
because I’M A POTATO”
It’s actually a copper-zink battery
Still enough to win the HaD prize. Just build a copper-zinc battery and use a fancy electrolyte, like algae, and you’re set!
Mark Rober attempted to charge a Formula E car with a lemon-array, but it wasn’t particularly effective: https://www.youtube.com/watch?v=a1D-fZP8qJk
You would need 1 crap-ton of lemons to really get som arcs going
All that fiddling about, and he still has the ‘Copper’ and ‘Zinc’ in a different font size!
About 15 years back, Xilinx released the Coolrunner CPLD, which we powered with grapefruits. The Coolrunner-II used a smaller process, so we used cumquats to power it. Both fruit batteries worked well with zinc nails and copper wire. The field apps engineers went at it and used beer with the nails/wire. Engineering customers loved it! I think my first exposure to fruit batteries was in ‘The Handbook for Boys’ – a Boy Scout Manual. Good fun!!
Is there any chance that you mean Kumquat, the fruit?