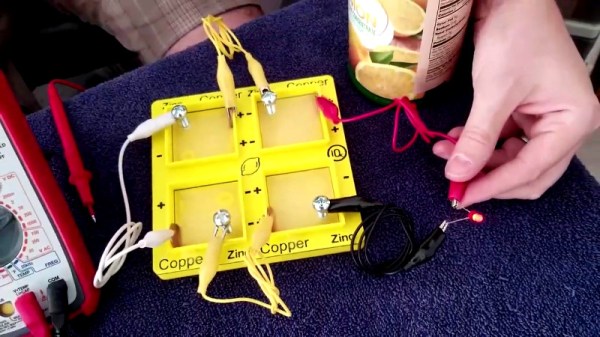
There are a whole bunch of high school science experiments out there that are useful for teaching students the basics of biology, physics, and chemistry. One of the classics is the lemon battery. [iqless] decided to have a play with the idea, and whipped up a little something for his students.
The basic lemon battery is remarkably simple. Lemon juice provides the electrolyte, while copper and and zinc act as electrodes. This battery won’t have a hope of charging your Tesla, but you might get enough juice to light an LED or small bulb (pun intended).
[iqless] considered jamming electrodes directly into lemons to be rather unsophisticated. Instead, an electrolyte tray was 3D printed. The tray can be filled with lemon juice (either hand-squeezed or straight from a bottle) and the tray has fixtures to hold copper pennies and zinc-plated machine screws to act as the electrodes. The tray allows several cells to be constructed and connected in series or parallel, giving yet further teaching opportunities.
It’s a fun twist on a classroom staple, and we think there are great possibilities here for further experimentation with alternative electrolytes and electrode materials. We’d also love to see a grown-up version with a large cascade of cells in series for lemon-based high voltage experiments, but that might be too much to ask. There’s great scope for using modern maker techniques in classroom science – we’ve discussed variations on the egg drop before. Video after the break.