We take the everyday materials of engineering for granted, as ubiquitous components rather than as complex items in their own right. Sure, we know that an integrated circuit represents the pinnacle of a hundred years’ development in the field of electronics, but to us it’s simply a black box with some wires. Even with more basic materials it’s easy to forget the work that goes into their manufacture, as for example with the two videos below the break. They both take a look from a very different angle at the creation of the same product: metal chain. However, the approaches couldn’t be more different as the two examples are separated by about a century and with vastly different techniques and material.
 The first film follows the manufacture of the chain and anchor that would have been found on a ship around the turn of the twentieth century. One of the text frames mentions Netherton Works, allowing us to identify it as being filmed at N. Hingley & Sons, a specialist anchor and chain manufacturer based in the area to the west of the English city of Birmingham known as the Black Country. It’s a window on a manufacturing world that has entirely disappeared, as large gangs of men do almost every task in the process by hand, with very few automated steps. There is scant regard for health and safety in handling the huge pieces of red-hot metal, and the material in question is not the steel we’d be used to today but wrought iron. The skill required to perform some of the steps such as forge-welding large anchor parts under a steam hammer is very significant, and the film alone can not convey it. More recent videos of similar scenes in Chinese factories do a better job.
The first film follows the manufacture of the chain and anchor that would have been found on a ship around the turn of the twentieth century. One of the text frames mentions Netherton Works, allowing us to identify it as being filmed at N. Hingley & Sons, a specialist anchor and chain manufacturer based in the area to the west of the English city of Birmingham known as the Black Country. It’s a window on a manufacturing world that has entirely disappeared, as large gangs of men do almost every task in the process by hand, with very few automated steps. There is scant regard for health and safety in handling the huge pieces of red-hot metal, and the material in question is not the steel we’d be used to today but wrought iron. The skill required to perform some of the steps such as forge-welding large anchor parts under a steam hammer is very significant, and the film alone can not convey it. More recent videos of similar scenes in Chinese factories do a better job.
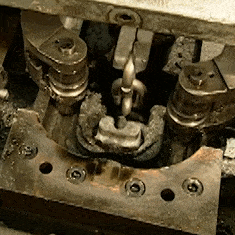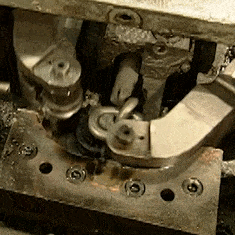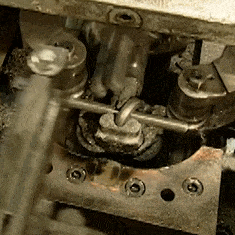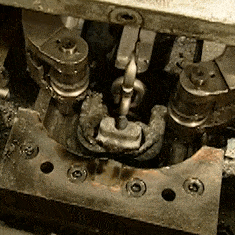 The other video is contemporary, a How It’s Made look at chain manufacture. Here the chains involved are much smaller, everything is done by automated machinery, and once we have got over marveling at the intricacy of the process we can see that there is far more emphasis on the metallurgy. The wire is hard drawn before the chain is formed, and then hardened and annealed in a continuous process by a pair of induction heaters and water baths. I’m trying really hard to avoid a minor rant about the propensity of mass-market entertainment such as this for glossing over parts of the process. A keen eye notices that each link has become welded but we are not shown the machine that performs the task.
The other video is contemporary, a How It’s Made look at chain manufacture. Here the chains involved are much smaller, everything is done by automated machinery, and once we have got over marveling at the intricacy of the process we can see that there is far more emphasis on the metallurgy. The wire is hard drawn before the chain is formed, and then hardened and annealed in a continuous process by a pair of induction heaters and water baths. I’m trying really hard to avoid a minor rant about the propensity of mass-market entertainment such as this for glossing over parts of the process. A keen eye notices that each link has become welded but we are not shown the machine that performs the task.
Most of us will never have the chance of a peek into a chain factory, so the medium of YouTube industrial films and videos is compulsive viewing. These two views of what is essentially the same process could not be more different, however it would be wrong to assume that one has replaced the other. There would have been mechanised production of small chains when the first film was made, and large chains will still be made today with fewer workers and from arc-welded steel rather than wrought iron. Plants like the Hingley one in Netherton may have closed in the 1980s, but there is still a demand for chains and anchors.
















How can there be no violent boiling and steam, as the orange-hot chain drops into cooling water at 2:16 in the second video?
Probably not water. Heated steel being “Quenched” often is dropped in oil.
You can quench mild steel in water just fine. It’s hard steel that you need to do an oil quench. Source: Watched YouTube
However the video sound track clearly, that stated water was used.
Possibly TV inaccuracy, it could be oil or even molten salt. (I have a small scar from the time I was doing metallurgy research and put a not-entirely-dry sample into a pot of molten sodium hydroxide. Which turns out to have many excellent properties as a quenching medium)
The Leidenfrost effect? There’s probably not much heat exchange going on until the chain has sufficiently cooled, and we aren’t shown that section of the chain.
Look closely as that orange-hot chain enters the water. There is boiling, but it is concealed by the rapid stirring of the water bath. The bath is stirred both to keep the temperature more or less uniform throughout, and to ensure that the hot steel is cooled quickly. Depending upon the steel alloy, a rapid quench may be essential. No doubt, fresh, cold water is constantly being added to the bath as well.
Please check for the full length segment of How It’s Made next time. They do show how the chain is welded at 3:12.
https://www.youtube.com/watch?v=w3ekikXZJtg
It is for comments like this that I wish Hackaday had a comment rating system. +1!
Always impresses me, the electric resistance welding, like spot welding that the part is a continuous loop of steel yet there is enough resistance through the extra 2 inches of length of chain link that electrons would prefer to travel between the link opening being pressed together. (i realize that they are traveling through both paths, and the smaller in cross section path is getting hotter.) Path of least resistance except when you stuff so many amps it doesn’t matter how little resistance there is.
I think the welding was the best bit!
And yes, it is way past time for a better commenting system…
I suppose those original films at 18 fps are gone now. It’s a shame each transfer degrades them more and more. For decades they kept up that speed it up stuff like it’s normal (18/24fps), then early video solarization, and then M-peg’s blocks. What a loss of a never again view of such noble work.
Well, the 18 to 24 fps conversion is easily reversed, and I would bet that anybody scanning old footage would archive at the highest quality available at the time. The fact that what you find on YouTube is often at very poor quality and resolution does not mean that the original quality has been lost permanently. Yes, many of the online digital archives are MPEG-1 and 320×240 or worse, but that’s at least partially because these have been scaled down and compressed down to make viewing them over the Internet practical — at the state of the Internet at the time the films were added to the archives. So the loss of quality over time may not be as bad as what you perceive.
Nitrate film stock was extremely flammable, and a lot of film was permanently lost to fires in storage vaults, so in many cases it was a matter of digitizing something at the then-current state of the art, rather than waiting for the technology to evolve and risk losing it altogether. And not just total loss due to fire, but also quality loss due to the deterioration of the base stock on which old films were printed, which is an issue even for later acetate prints.
Nitrate film was so dangerous, the Library of Congress would not accept nitrate film, and many films were archived by making a print onto paper stock, which was not only safer, but also deteriorated slower than either nitrate or acetate films. When you see a film from the 1920s today that looks like new, it’s probably because the new print (or digital scan) was from one of these paper prints.
Of course, nearly everything from our contemporary culture is doomed to be lost, due to the fact that nothing today is being archived on long-term-stable media. Sure, disk drives using magnetic oxides on glass are pretty durable, but the electronics needed for reading them not only isn’t as durable, but is in most cases proprietary. The first thing that will go is the firmware, which is mostly on flash ROM, which only holds a charge for a few decades. So when somebody in the year 2200 gets a pile of disk drives, what are the chances he’s going to be able to read them? And worse than that, there are so MANY disk drives, all of which look the same, how would he know which ones contain high-quality original content, and which just have low-bitrate rips from DVDs? Archiving only works as long as the archive is being maintained, and while we now see some value in industrial films such as the first video in this article, this film is probably available to us only by luck, having been left in a roomful of films that miraculously never burned down over the many decades, and was recorded on a format (16 mm film) that turned out to be an enduring standard that can still be viewed and scanned today.
TL;DR: Sic transit gloria mundi.
But on nitrocellulose film: Friend of mine has an annual “physics picnic” (Hi Tommy) and one year we set off some old film on a fireproof slab in the middle of a field. The rumors of its flammability are true. I wouldn’t say it was explosive, but it was certainly very accelerated.
to mess with the temper or harden steel (mild steel isn’t hardenable besides case hardening, not enough carbon)… “O” varieties of tool steel you quench in oil, “W” you quench in water, and “A” you quench via air. There are some other ones which are more fussy, and some that work better in a pinch. Some are quenched in a giant liquid salt bath.
Jenny, I have always marveled at the genius engineering involved in automation in manufacturing. It not only has to work once, it has to be repeatable and be dependable to keep costs down. I have only scratched the surface of this in my career but, to this day, watching something as “simple” as chain being made still astounds me. Who thought of that? Who funded the creation of the machinery? Who purchased these machines? I thank you for this inspiring look back at the automation of manufacturing something “simple”.
I can wrap my head around “who thought of that?”. It’s simply really. If you spend time making a thing over and over again, most will try to find ways to minimize the work. It happens gradually, a tube to wrap wire around to form coils, a guillotine to chop the coil into rings, a press to close the ends of the rings in bulk, a pot of solder to dip them in to join the ends and provide a shiny finish and on and on until all that’s left for the human is to swap out input materials and collect output materials. The level of automation achieved is going to be proportional to the amount of time one or more humans spend performing that task.
The one I have trouble with is “who funded the creation of the machinery?”. Employers don’t give a crap about how inconvenient a task is for the human to perform. Employers overly valuing human labor over machine labor. A variety of reasons get in the way. Yet somehow it happens.
Exactly my point. I have designed and built a few machines for our manufacturing use but it takes all 4 of my above points to have a machine setting in a plant somewhere working away like this all over the world. The idea, the funding/time to build it and perfect it, then deciding to manufacture it and last, selling them to end users. I will always be amazed by this.
I agree with your amazement, but it’s also the oldest story in the books. Shoulders of giants, incremental progress, and all that.
Imagine you were a blacksmith, and you spent your whole day hammering. Hard work. Then you’re like, “hey, the miller over there doesn’t turn his grain-grinding wheel by hand, he lets the water do the work” and then you’ve invented the trip hammer (or whatever it’s called). A few centuries later, and you get tired of bending the chain links by hand too.
What is astounding is the acceleration of these generations in the industrial/post-industrial era. Shared info. Tool-making tools. Etc. But it’s not like some one dude was like “how do I make a chain factory?” et voila.
But yeah. The depth of human knowledge and ingenuity across nearly every field is astounding.