You don’t really create energy, you convert it from one form to another. For example, many ways that we generate electricity use heat from burning or nuclear decay to generate steam which turns a generator. Thermocouples generate electricity directly from heat, but generally not very much. Still, some nuclear batteries directly convert heat to electricity, they just aren’t very efficient. Now researchers have developed a way of preparing a material that is better at doing the conversion: tin selenide.
Tin selenide is known to have good performance converting heat into electricity when in its crystal form. However, practical applications are more likely to use polycrystalline forms, which are known to have reduced conversion performance.
The material works well because it is not very thermally conductive and it has a favorable band structure that allows multiple bands to participate in charge transport. However, in polycrystal configurations, the results are not as good due to higher thermal conductivity. Yet crystalline tin selenide is difficult to manufacture and not very robust in real-world use.
The team worked out that the polycrystal material’s thermal properties were due to tin oxide films on the surface. Using a particular method of construction, you can remove the tin oxide and improve performance even better than the crystal version of tin selenide.
Creating this material might be beyond your garage lab, though. You need a fused silica oven that can reach a pretty tight vacuum. Although you might be able to swing it. Otherwise, you might stick with more conventional methods.

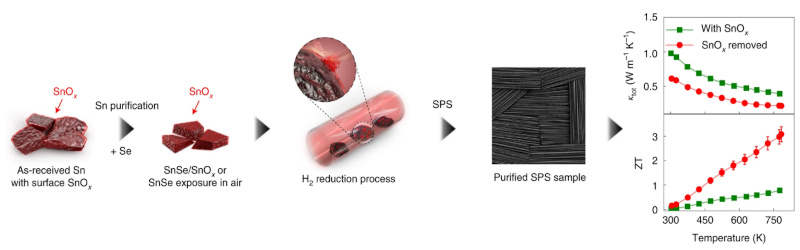














I skimmed through the article to understand this a bit better. The problem with the tin oxide is that it completely surrounds each grain of crystal, effectively making a thermal short circuit around the crystal. This means that the crystal has very little temperature gradient to make electricity from.
What I couldn’t find or figure out is a number for the efficiency of conversion from heat to electricity. Can anybody say what this might be?
That you want is the ZT value near the top of the linked article. “ZT of roughly 2.2–2.6 at 913 K” for tbe polycrystalline version and about
“3.1 at 783 K” for the polycrystalline version of the oxide layer is removed.
This article might make for an explanation of what the ZT number is. [ But it’s essentially the efficiency, akin to the Q factor of an inductor. ]
http://thermoelectrics.matsci.northwestern.edu/thermoelectrics/
The equation for ZT is here:
https://en.wikipedia.org/wiki/Thermoelectric_materials#ZT
The summary of which would be lower thermal conduction is better, higher electrical conduction is better, and higher Seebeck coefficient is better.
And the equation here can give you the maximum efficiency of a thermoelectric device knowing only ZT with the hot and cold temperatures.
https://en.wikipedia.org/wiki/Thermoelectric_materials#Device_efficiency
Thanks. For ZT = 3.1, Th = 700 K, Tc = 350 K, the formula gives maximum theoretical efficiency of Carnot_limit * 0.405, or 20.25%. My dim recollection from 20 years ago is that thermoelectric generators are much worse than this, so this new tech is a nice advance.
It’s always surprised me that in the 21st century we still get most of our energy from what are basically giant kettles.
Hackaday: “You don’t really create energy, you convert it from one form to another.”
Nuclear Engineers: “cough“.
Yes, I’m being a jerk; acknowledged. :-)
Nuclear energy comes from the breaking of the weak nuclear force, ie. one form of energy converted to another.