Sometimes it begins to feel like a tradition that a certain substance or group of substances become highly popular due to certain highly desirable chemical or physical properties, only for these chemicals then to go on to turn out to form a hazard to the biosphere, human life, or both. In the case of per- and polyfluoroalkyl substances (PFAS) it’s no different. Upon the discovery that a subgroup of these – the fluorosurfactants – have the ability to reduce water surface tension significantly more than other surfactants, they began to be used everywhere.
Today, fluorosurfactants are being used in everything from stain repellents to paint, make-up, and foam used by firefighters. In a recent study of 231 cosmetic products bought in the US and Canada (Whitehead et al., 2021), it was found that all of them contained PFAS, even when not listed on the packaging. The problematic part here is that PFASs are very stable, do not decay after disposal, and bioaccumulate in the body where they may have endocrine-disrupting effects.
Some areas have now at least partially banned PFAS, but the evidence for this is so far mixed. Let’s review what we do know at this point, and which alternatives we have to continuing to use these substances.
Love It or Hate It
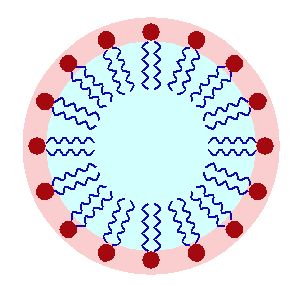
Surfactants (surface-active agents) find uses in wetting, dispersing, emulsifying, foaming as well as anti-foaming agents. This versatility has resulted in them making it into an astounding number of products, ranging from personal care items including shampoos, conditioners, cosmetics, and toothpaste, to ski waxes, anti-fogging treatments, inks, adhesives, paints, soaps, emulsions, fabric softeners, and detergents, to firefighting foam, herbicides and insecticides.
The basic principle that makes surfactants work are a head that’s hydrophilic and one or more tails that are hydrophobic. This enables the macro properties like foam control or emulsification that form such an essential feature of many every day products.
Most surfactants’ tails are rather similar, taking the form of a hydrocarbon chain. PFAS used as surfactants have a fluorocarbon chain instead, which offers better properties than hydrocarbon-based surfactants, in addition to their better stability in harsher environments. This stability also explains why discarded PFAS don’t degrade, but instead collect in surface and ground water, as well as in the soil and in the bodies of animals – including humans.
PFAS Everywhere Around You
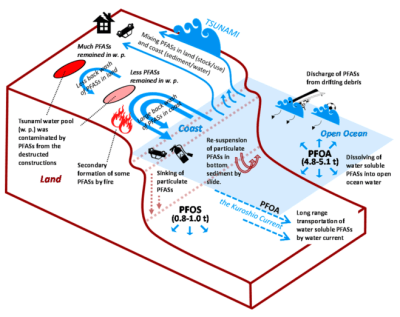
from land to ocean following the earthquake EQ 3.11. (Credit: Yamazaki et al., 2015)
When the massive earthquake and subsequent tsunami near Fukushima, Japan, hit, it caused both massive destruction and the release of large amounts of chemicals into the environment. PFAS were among these chemicals, and these were tracked in a 2015 study (Yamazaki et al.). This event could be regarded as a time-accelerated version of the usual spread of PFAS.
Tracked were primarily PFOS (perfluorooctonatesulfate, C8HF17O3S ) and PFOA (perfluorooctanoate, C8HF15O2), which are used widely in carpeting, floor waxes, and sealants. These and other PFAS were measured in 2010 and again in 2011 in the ocean waters.
This study shows how rainwater carries PFAS from land to surface waters, with ocean currents like the Kuroshio Extension Current apparently transporting PFOA and PFHxA, but not PFOS and PFHxS based on measured levels. This indicates that different types of PFAS do not diffuse equally in the oceans, and hint that the same might be true elsewhere. Yamazaki et al. speculate that this might be due to the different water solubility of the PFAS types.
In a less disastrous setting, PFAS find their way into surface waters via sewer systems, landfills and rainwater, with some amount being ingested by animals and biomagnification ensuring that the total amount of PFAS in each subsequent predatory creature increases. As PFAS like PFOS tend to accumulate in the liver (Jones et al, 2009), and bind to serum proteins, the likelihood is high that they will find their way up in the food chain.
The Human Impact
PFAS, being chemically inert, was assumed to be biochemically safe. The exact impact on human health is still being assessed today. One of the biggest studies in that regard was the C8 Health Project, which saw 69,030 participants enrolled. These participants lived in an area with a large contamination with PFOA (also referred to as ‘C8’ here). The findings were summarized by Steenland et al., 2020.
They found a supportive association with kidney and testicular cancer exists, though there is no evidence of other site-specific cancers. A positive association with cholesterol is consistent, and there’s evidence for ulcerative colitis, but not other auto-immune diseases. As noted by Steenland et al., the epidemiological evidence remains limited, even in such a large meta-study.
Solid evidence exists in the effect of PFOA and PFDA (perfluorodecanoic acid, C10HF19O2) downregulating activity in the liver, as described by Cheng et al., 2008, in mouse livers. Both PFASs are agonists for the PPAR-α receptor, the effect of which is the downregulation of mRNA expression for polypeptides that are required for the uptake of bile acid (BA). There are a number of negative associations with increased BA levels, which was found for PFDA but not PFOA, although both are clearly affecting the liver.
Whether or not male fertility is affected still needs more research (Tarapore et al., 2020), while the question of food safety has been studied by the European Food Safety Agency, which has set maximum allowed daily intake levels for PFAS based on their findings. They note studies (e.g. Macon et al., 2011; Tucket et al., 2015; White et al., 2011) that indicate clear negative impacts of PFOA on the development of the mammary glands of animals exposed in utero, during lactation, etc.
Also noted are the observed effects on the body’s immune system. What makes it hard to establish definite causality, however, is that the mechanism behind various adverse effects is still unclear. This makes it hard to impossible to make any definite statements about how bad each type of PFAS is, leading to a cautionary approach that also ties into the search for alternatives.
Alternatives
The use of PFOS has been reduced significantly already. For example, 3M has replaced PFOS with the shorter chained perfluorobutanesulfonic acid (PFBS, C4HF9O3S) in Scotchgard. Where PFOS has a half-life of 5.4 years in humans, PFBS sticks around for roughly a month. Whether is shorter half-life in the body is sufficient to allay any potential health effects is still unknown, and the European Union has added PFBS to the candidate list of Substances of Very High Concern (SVHC) as a result.
The impact of PFAS continues to be studied by the EPA, as well by the Canadian government, with no immediate timeline for action. A program to investigate the use of shorter chain PFAS as alternatives has been submitted for review.
While there has been some evidence suggesting that longer-chain PFAS are involved in negative health effects, both for humans and animals in general, we lack an understanding of the mechanisms behind these effects. The amount of PFAS in the environment is unlikely to decrease soon, and it’s too early to say whether shorter-chain PFAS are an actual fix here (Birnbaum et al., 2015). This leaves us in an uncomfortable limbo.
Yes, No, Kinda
As unsatisfactory it is to be left without a clear and absolute conclusion on whether PFASs in general are good or bad, the reality remains that this is a complex topic, involving many chemicals and countless, complex interactions. While some studies have shown clear evidence that some types of PFAS like PFOS and PFOA are harmful, many of the effects are perhaps not strong enough to be noticed against the background of everything else that our environment subjects our bodies to.
Perhaps the obvious course is to apply the precautionary principle, and use alternatives to PFAS where we can, and PFASs that degrade faster when we can’t, even if we cannot be certain that the alternative is perhaps not worse than the original. This, as noted in the introduction, remains the eternal problem with assessing the safety of chemicals in the environment and in our bodies: we can only do our best using the knowledge and technology we have today.
[Banner image: “Water droplets on hydrophobic feather!” by The Manic Macrographer, CC BY 2.0. (Feathers do it with nanostructures instead of fluorines.)]
[Thumbnail image: “A water droplet on a coated surface” by Brocken Inaglory, CC BY-SA 3.0]















Apparently all the data for those cancer cases came from http://dx.doi.org/10.1289/ehp.1306615 and had no extra data confirming it afterwards. Taking a look at the paper, it looks like a fishing expedition: they looked at the numbers for 21 types of cancer, and those 2 (testicular and kidney) are the only ones that had usable p numbers…well, else that it also indicated a LOWERED chance of breast cancer, but who would follow up on that?
Also of note is that they had a total of 19 cases of testicular cancer in the 32254 total population, so any claims coming out of that should be taken with a block of salt.
Thanks for digging into it. I appreciate the quality of the article above, but I feel like this kind of commentary should be what the conclusion of this article should have been.
No? The article soft-pedals the safety of PFAS so hard I thought it was a piece paid for by 3M.
The author repeatedly implies that evidence of harm is nebulous…right after mentioning –numerous– studies showing all sorts of health effects.
So, completely out of my area of expertise here and trying to understand the whole fishing expedition thing.
I can understand that if you look at a large enough pool of data you’re bound to find at least a couple correlations but wouldn’t that also be expected outcome if there actually was a cause and effect link between the two? Like if I looked at 21 types of cancer and tried to find a link to sun exposure I should expect to find usable p values only for skin cancer right?
A p value is, roughly speaking, the chance that some correlation is due to random chance. The usual standard (the same used on the paper) is p <= 0.05, which is a 5% chance of that result being just a coincidence. IIRC one of the results from the paper has a p of 0.04, don't remember the other one, so that one has a 4% chance of being just a random match.
A "fishing expedition" is generally when you don't know what you're looking for, or didn't find what your main hypothesis was, and just start checking for all correlations and looking at p values that fall on the <= 0.05 range so you can publish something. Generally, if you're looking at 21 different items with weak correlations, you're still likely to find at least one with p <= 0.05.
The number of samples also weighs heavily here. Their universe looks reasonable at a first glance with 32254 samples, but the incidence of those cancer types is so low, that this number becomes insufficient to give enough resolution for you to see actual increases in the cancer rates. The testicular one had just those 19 confirmed cases, and the "increased" rate was around 10%, so about 2 extra cases than expected, which I would say is way into the expected random variation for it. The kidney one is more prevalent, but it only had a bit over a hundred cases in the area.
The main problem with this kind of low-frequency analysis is that you need a LOT of data to be certain of anything, and with low data telling noise or real effects apart is almost impossible. The best we can say in this case is "inconclusive" and "needs more study", the effect is definitely small enough for any real concern.
“Generally, if you’re looking at 21 different items with weak correlations, you’re still likely to find at least one with p <= 0.05."
I'll just leave this here: https://xkcd.com/882/
Should have remembered that one myself and saved a couple paragraphs XD
The “linguistically interesting”, but “Still Not Significant” list:
https://mchankins.wordpress.com/2013/04/21/still-not-significant-2/
People have been tempted to compare the ratio of occurrences with circular-cited peer-reviewed papers.
Try it, and one will certainly never make tenure…
;-)
Tenure decisions are not infrequently based on fear of losing “F&A” (the percentage the university is allowed to skim) from the Assistant Professor’s revenue stream. At least in heavily research-oriented departments – English, for instance, is a very different story. If the Discounted Net Present Value of future cash flows exceeds your salary, it’s in the department’s interest to keep you as long as possible.
Circular (or more like “cascading”) citation is the rule in smaller disciplines because there is neither enough scholarship to avoid it nor enough money for replication.
Thank you. I would replace the whole Wikipedia page on p-value by your first paragrph.
Local practices matter – this is (probably!) why the kidney dialysis five year survival rate varies so much across the US. I would be interested in knowing if the local doctors are biased toward certain drugs. We see this a lot in the US. It would be very interesting to see if a few local doctors preferred, say, lithium over valproates a little more than typical.
If you’re curious, I can thoroughly recommend “Bad Science” by ben Goldacre, it’s very readable and engaging and explains all sorts of shady scientific methods and practices, and just generally how to spot when you’re being bullshitted.
i grew up near a major manufacturer. the evidence is in the lack of life and deformed animals that now live there. there was a massive decrease in wildlife starting directly after the plant showed up. coincidence? ya. i dont care. id rather have my damn farm back and be able to hunt again and catch crafish that are now so rare compared to the huge buckets we used to find and catch. so you will forgive me if i would rather err on the side of caution and just let people use other products that dont fuck everything up.
One dire prediction of mine is that all of our convenience driven assaults on nature will result in the soil becoming hydrophobic and not supporting the growth of plants and fungi that nourish the the soil. Then Earth will join Mars as a place to try to reinvent what we will lose. It’s appalling that cosmetics-makeup is on this action list. Forest fire suppression chemicals are a good start to bring it all down.
That said, even if we continue to screw up Earth really bad, it’s still way more hospitable than Mars. Less idyllic, but still way better.
+10 The slip-n-fall lawyers are preparing the Facebook ads “If you or anyone in your family every used cosmetics and got cancer…” All they need is one carefully selected jury of morons and the $billions we be there for the taking.
I can recommend the episode of “last week tonight” with John Oliver about PFAS from 04.Oct.2021. Its on YouTube.
-> https://www.youtube.com/watch?v=9W74aeuqsiU
And if I recall correctly he/they named studies by Dupont made in the 60s and 80s which already indicated negative effects to health / nature and what-not.
The movie Dark Waters is about the case mentioned in Ohio where Dupont was producing teflon and PFOA. A decent watch.. https://en.wikipedia.org/wiki/Dark_Waters_(2019_film)
https://youtu.be/-pW2ATrDnA8
This video about this subject has just popped in my yt feed.. Coincidence?
If you’re using Chrome or Android, and are logged into your google account, then of course YouTube knows what you’re browsing using google’s other personal monitoring tools.
I don’t know if this is a coincidence but currently in my country, Belgium, a whole scandal is going on. Around Antwerp, one of the major cities in the Dutch speaking part (flanders) has discovered PFOS everywhere during road travels. It has turned out this came Frome a nearby 3M factory.
… and after investigating a 15 kilometer radius around the factory, it was determined that everywhere within that 15 kilometer radius it was advised not to consume vegetables or eggs from your own garden.
The kicker? They never investigated beyond the 15 kilometer radius.
I would be interested in scientific or governmental reports on that, if you can find some. I think I might miss some of I just googled, since local/regional knowledge and background knowledge massively helps to find such stuff in cases like this, in my experience.
News reports are welcome, but I can find those myself, I think.
Here’s an English version of a report on the PFAS pollution in Flanders, commissioned by the Flemish government: https://publicaties.vlaanderen.be/view-file/46454
PFOA and PFOA have not been used in carpet since around 2005.
The permissible limits for PFAS are ridiculously low for a great deal of environmental protection guidelines. The drinking water guideline in a certain Australian jurisdiction was, until recently, about 70 parts per trillion. Remember that one part per trillion is roughly one drop in four Olympic-sized swimming pools. We’re talking homeopathic quantities of PFAS here. The detection limits of the instruments I’ve used to test for PFAS is about one tenth of a part per trillion. The main concern isn’t its toxicity – most of the tox data is sketchy at best and the precautionary principle is being liberally applied here. Some of the PFAS are definitely worse than the others, the rarely-seen PFDA for instance.
The funny thing about most PFAS species is that there’s no obvious mode of action for toxicity. Ask an organic chemist with a pharmacology background (like myself) their opinion on a molecule that looks like PFOA and chances are they’ll say ‘it won’t do anything’. The most promising pathway for toxicity is if a perfluorinated compound lodges into a cell membrane, messes up the permeability of the membrane due to its high surface energy, and starts letting stuff into the cell that it shouldn’t. You’d really need to be swimming in the stuff to see those kinds of effects to the point where they can’t be naturally overcome by your body’s protective pathways.
The main issue is that it accumulates and it’s really hard to break down. I think the gold standard in PFAS remediation at the moment is to incinerate the soil at about 900 deg. C, and that doesn’t even get rid of all of it. But, now that we’ve had a phase-out of most PFAS materials (I’m sure that Chemours is still pumping out some weird cocktails that haven’t been blacklisted yet) hopefully that level doesn’t increase too much. But in terms of background levels, PFASes are generally pretty benign and I wouldn’t worry too much about them. Sometimes I think the ever-decreasing permissible limits for PFAS are just a way for the mass spectrometer companies to spruik the fact that their newest instruments can resolve an infinitesimal whisper of PFAS in a vast cavern of contaminated water…
I do wonder how much of the negative effects attributed to PFAS in firefighters are caused by or correlated with PFAS. It’s a dangerous profession and you’re well within risk areas where you’d be exposed to all kinds of nasties like benzo(a)pyrene.
> We’re talking homeopathic quantities of PFAS here.
>The most promising pathway for toxicity is if a perfluorinated compound lodges into a cell membrane
Both statements imply a moderate-concentration, low-specificity mode of action. It would be prudent not to discount pathways that are highly specific in nature and can cause changes in low concentrations, as it seems to be the case in gene expression.
Here’s a quick find searching for that: Xu et al. (2020), “Association between serum concentrations of perfluoroalkyl substances (PFAS) and expression of serum microRNAs in a cohort highly exposed to PFAS from drinking water” https://doi.org/10.1016/j.envint.2019.105446
“Three microRNAs were consistently associated with PFAS exposure in the different steps of the study: miR-101-3p, miR-144-3p and miR-19a-3p (all downregulated with increasing exposure). In silico functional analyses suggested that these PFAS-associated microRNAs were annotated to e.g. cardiovascular function and disease, Alzheimer’s disease, growth of cancer cell lines and cancer.”
>limits for PFAS are ridiculously low for a great deal of environmental protection guidelines
“ridiculous” is an interesting term. As we learned from other substances we exposed ourselves to and released into the environment, the amount of evidence for the proof of harmlessness should not be less sound than evidence demanded to flag a chemical as harmful. We’ve got enough “ignorant until proven guilty” already.
California is proposing public health goal for PFOS in drinking water 1 ppt (part per trillion) and for PFOA at 0.007 ppt. That’s 7 parts per quadrillion. These public health goal numbers trickle down to enforceable policy limits in other agencies.
https://oehha.ca.gov/water/crnr/announcement-availability-draft-technical-support-document-and-public-workshop-proposed
PFOS was also the active component in fume suppressants required to control air emissions from chrome plating.
Something is very fishy here – a HaD article with a (very) well-informed and civil comments section?
Is your point that human cancer is the only harmful outcome we should be concerned about?
Paragraph 8 ‘PFOS perfluorooctonatesulfate’ should be -sulfonate.
We’ll have to wait until credible studies are done. Meanwhile, let’s keep using our pfas shampoos. Like we did with ddt shampoos to combat lice during wartime.
“Perhaps the myth of the harmlessness of DDT rests on the fact that one of its first uses was the wartime dusting of many thousands of soldiers, refugees, and prisoners, to combat lice.” (Rachel Carson, Silent Spring 1962)
Phase change cooling liquids (fluorinerts) became very popular in the last years (even in DIY PC and miner scenes). I was always concerned about its’ health effects because its are 99.5% pure, but they don’t let you know what is the remaining 0.5%. For example HFE 7000/7100.
It’s not completely clear to me what the advantages are if a bioaccumulating “forever” substance has been modified to a thirty day or five year half life, if exposure is daily and perpetual.
The bio-accumulated amount depends directly on the average intake and half-life of it. A bio-accumulating “forever” substance would have effectively infinite half-life, and the amount present in the organism should be just the sum of all the intake over the lifetime of the organism. A substance with a half-life of 5 years will peak at around 2600 times the daily intake, and half-life of 30 days will peak at around 44 times the intake.
Like they say, “the dose makes the poison”, so the half-life is very important, but it’s just another factor you have to take into account when playing with stuff like this. What will do more harm, a high concentration of a harmless substance, or a tiny concentration of a highly poisonous one?
2022-09 new documentary on the subject of PFAS (German, auto-translated subtitles)
“Jahrhundertgift: Warum wird es nicht verboten? | STRG_F”
https://www.youtube.com/watch?v=ovCvW22ol3Y
Bloomberg: “The Toxic Legacy of 3M’s ‘Forever Chemicals'”
https://www.youtube.com/watch?v=5CUvPykRxHI
Another piece from ARD:
“A poison that lasts for centuries: PFAS found in more than 1500 sites in Germany” /
“Jahrhundertgift: PFAS an mehr als 1500 Orten in Deutschland nachgewiesen”
“we must stop now. It’s already in the food chain. We’re way behind the reality of industrial use when it comes to analysis and monitoring.”
https://www.youtube.com/watch?v=UA4VXK0BoN8
The Climate Show with Tom Heap, touching on the US proposed change of PFAS limits in drinking water – dropping from 100 ppt to 4 ppt. Limits also to be considered in the UK.
Segment starts 5:52
https://www.youtube.com/watch?v=sf4TdZhAbNM
“Contaminated drinking water: Netherlands hold 3M liable
3M has discharged chemical waste into the Scheldt. After penalties from the Belgian government, the Netherlands now also want to prosecute 3M.”
https://www.heise.de/news/Ewigkeits-Chemikalien-Niederlande-machen-3M-haftbar-9064679.html (German article)
Don’t mind me keeping track of the subject as time goes on. There certainly is a new sense of awareness and action.
“How 3M And DuPont Are Being Sued Over ‘Forever Chemicals’ In Water”
https://www.youtube.com/watch?v=UBEjgIyp4FA
Great video by Reactions on the hydrothermal treatment of PFAS – that is, not by tossing everything into an incinerator. “Super-critical water oxidation”:
https://www.youtube.com/watch?v=eglcqP2qv1w
This keeps making waves – “The Forever Chemical Scandal | Bloomberg Investigates”
https://www.youtube.com/watch?v=t8qGtEVh7oQ
Interesting new report by BR – “we need an everyday chemicals transition”
[tip line] Quo vadis, PFAS? Moloch demands we keep producing PFAS to stay competitive, yet in the long run, accumulation in the environment means agriculture will get less productive and eventually more wells will need to be closed due to contamination, further impacting drinking water availability.
“PFAS – Gift für die Ewigkeit | Wie abhängig sind wir? | Gefährlich und praktisch | ARD Wissen | BR”
https://www.youtube.com/watch?v=qatBi2ezfTo
Kudos for keeping track of some of the media on PFAS.
Sidenote: I wanted to buy a new pan, and tried to steer clear of fluoride chemicals. Got sold a WMF pan, explicitly asking at the time if PFAS were involved in the non-stick surface. The seller said there wasn’t. Turns out: that is correct in regard to the application process, which doesn’t use PFAS. But the non-stick surface still contains PFAOs – which I found out much later.
There is currently indeed some movement, which is quite interesting. It only took decades to take action.
Oddly enough, Now that attention has been drawn to PFOA (perfluorooctanoic acid), some manufacturers have switch to, or continue to use PFOS (perfluorooctane sufonic acid), while others market their products as “PFOA/PFOS-free”, but may still use other, more obscure PFAS.
Whether they indeed have less troublesome biological effects and higher excretion rates remains to be seen. But indeed, it will be interesting to see whether switching to alternatives causes less problems or just a legal loophole.
The Guardian article pointing out study results that show 2-5 orders of magnitude higher concentration of PFAS in ocean spray, exposing an aerosolization of PFAs compounds which hadn’t registered as a major source of exposure and atmospheric pollution.
https://www.theguardian.com/environment/2024/apr/19/ocean-spray-pfas-study
PFAS-contaminated ground water can be processed by applying multiple stages of foam fractionation, allowing PFAS molecules to be carried to the surface through an, if you like, continuously replenishing air-water surface conveyor belt onto which they arrange.
“A review of foam fractionation for the removal of per- and polyfluoroalkyl substances (PFAS) from aqueous matrices”
doi: 10.1016/j.jhazmat.2023.133182
The paper linked in the article:
“Constraining global transport of perfluoroalkyl acids on sea spray aerosol using field measurements”
doi: 10.1126/sciadv.adl1026
One of the PFAS crime scenes I’m following the news on is in southern Germany, where 100,000t of contaminated paper industry waste had been mixed in with compost and “given out for free” to farmers. Nobody questioned it. The municipality has been dragging their feet and ultimately given the fourteen paper mills on the list of suspects a free pass. The perfect crime.
“ZDF – Schweres Umwelt-Verbrechen: Gefährliche Chemikalien im Trinkwasser | Umwelt Crime”
https://www.youtube.com/watch?v=DTxP4m9Otrs