Water takes a lot of energy to heat up. If you’d like evidence of this, simply jump into a 50° F swimming pool on Memorial Day. Despite the difficulty of heating water, that simple act accounts for a lot of industrial processes. From cooking a steak to running a nuclear reactor, there isn’t much that doesn’t involve heating water.
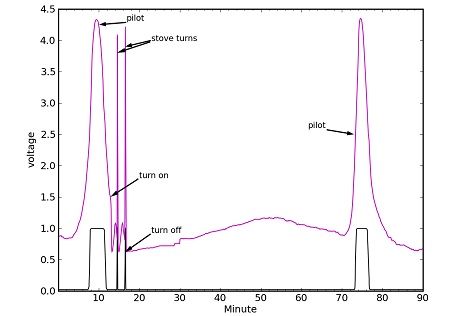
[Tom Murphy], Physics prof at UCSD decided to test out exactly how efficiently he could boil water. Armed with a gas stove, electric kettle, microwave, and a neat laser pointer/photodiode setup on his gas meter to measure consumption, he calculated exactly how much energy he was using to make a cup of tea.
The final numbers from [Tom]’s experiment revealed that a gas stove – using a pot with and without a lid on large and small burners – was about 20% efficient. A gas-powered hot water heater was much better at 55% efficiency, but the microwave and electric kettle had a miserable efficiencies of around 15 and 25%, respectively. There is a reason for the terrible inefficiency of using electricity to heat water; if only the power from the wall is considered, the electric kettle put 80% of energy consumed directly into the water. Because the electricity has to come from somewhere, usually a fossil-fueled power plant that operates at around 30% efficiency, the electric kettle method of turning dinosaurs into hot water is only about 25% efficient.
The take-home from this is there’s a lot of power being wasted every time you run a bath, make some coffee, or wash the dishes. We would all do better by decreasing how much energy we use, much like [Tom]’s efforts in using 5 times less power than his neighbor. Awesome job, [Tom].
![The hydrogen-powered Honda Clarity FCV, a car most of us will probably never see. Lcaa9 [CC BY-SA 4.0].](https://hackaday.com/wp-content/uploads/2018/12/1024px-2018_honda_clarity_fuel_cell.jpg?w=400)














