A bunch of audio heads over at the Head-Fi forum were discussing handy and quick heat sinking methods, leading to much speculation and conjecture. This finally prompted [tangentsoft] to take matters in his own hands and run some tests on DIY Heat Sinks.
The question that sparked this debate was if a paper clip is a good enough heat sink to be used for a TO220 package. Some folks suggested copper pennies (old ones minted 1981 and earlier – the new ones are zinc with copper plating and won’t help much). [tangentsoft] built a jig to test six LM317 regulators in constant current mode set to 0.125A and 2w dissipation. The six configurations were a paper clip, a single penny bolted to the regulator, a regular Aavid TO220 heat sink, a set of 4 pennies bolted, a single penny epoxy glued and finally a single penny soldered directly to the regulator.
The results were pretty interesting. The paper clip scored better than any of the single pennies! The quad-penny and the Aavid heat sink fared above all the other configurations, and almost at par with each other. [tangentsoft] posts his review of each configurations performance and also provides details of his test method, in case someone else wants to replicate his tests to corroborate the results. He tested each configuration independently for one hour, gathering just over 10000 readings for each setup. Other nearby heat sources were turned off, and he placed strategic barriers around the test circuit to isolate it from the effects of other cooling / heating sources. He even removed himself from the test area and monitored his data logging remotely from another room. When he noticed a couple of suspect deviations, he restarted the test.
[tangentsoft] put all the data through Mathematica and plotted his results for analysis, available at this link [pdf, 2.8MB]. So the next time you want to heat sink a regulator for cheap, just hunt for Clippy in your box of office supplies. Do remember that these methods will work for only a couple of watts dissipation. If you would like to cast and build your own heat sinks out of aluminum, check out this post about DIY Aluminum heat sink casting. And if you need help calculating heat sink parameters, jump to 12:00 minutes in this video from [Dave]’s EEVBlog episode on Dummy loads and heat sinks.
Thanks to [Greg] for sending in this tip.

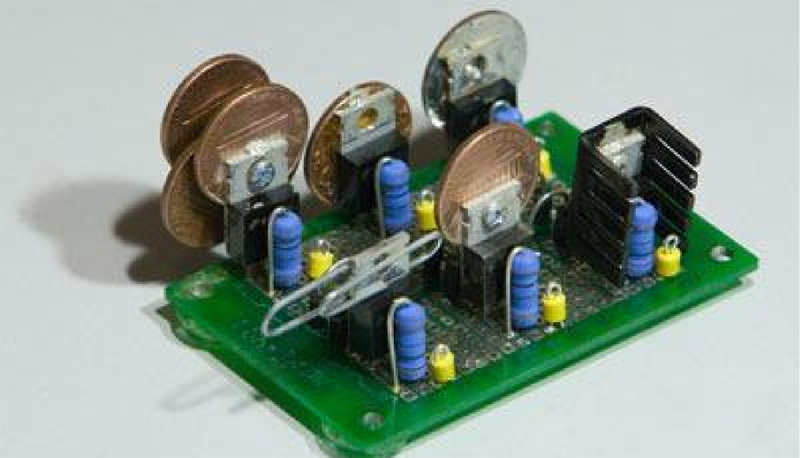













Very thorough work, but why do you put six of them on one board in such close proximity?
The masses of the next neighbor also absorb thermal radiation energy.
Agreed, this is a huge problem when performing controlled tests, also ambient temperature and even to some extent humidity will affect results.
It is no surprise that the winners were on the four corners.
Interesting!
However, I do believe that the soldered penny and the epoxy glued one could get better results if they were oriented the same way as the bolted one. I guess he did it that way because it was painful enough to fix the damn things together.
u am not sure how legal using pennies are since it is destruction of federal property.
copper was and still used for heat sinks and you can go to the hardware store and get copper strips used for crafts or a plumbing store and maybe they will give you some short scraps of plumbing scraps and the good part is this is a lot more legal than using pennies.
“u am not sure how legal using pennies are since it is destruction of federal property.”
Right, like anyone really cares about that.
“u am not sure how legal using pennies are since it is destruction of federal property.” Check your federal laws, not true everywhere. “fraudulent” alteration is illegal in the US, and I assume that’s his case since he’s using American pennies…
Right, nobody gets busted for using a novelty penny-smusher machine, or putting coins on a train track.
Or if you were melting down massive amounts of pennies because the base metal was worth more than the coins themselves, that would also be illegal. This nearly became possible at one point due to the soaring price of copper, prompting the US to switch to copper-plated zinc pennies. (So it’s no surprise these pennies performed poorly as heatsinks, unless old pennies were used, and I see no note of this.)
Basically, it’s a broadly worded law they only bother to enforce when there’s a good reason to do so.
They’re interested in misrepresentation and fraud (eg gold plating a coin and selling it as solid gold) and copyright infringement (you own the coin, they own the design):
FAQ here: http://www.usmint.gov/consumer/?action=FAQ
He did select pre-1982 pennies.
“All of the pennies are 1981 or earlier vintage, that being the last year US pennies were made mostly of copper. After that, they’re mostly zinc, with some copper plating. The pennies in configurations 2, 5, and 6 are all 1981 pennies made in the same mint. I didn’t feel a great need to find identical pennies for configuration 4.”
So they should be solid copper.
Using pennies for science is completely legal. You can smash them in presses, removing their original markers, you can melt them with concentrated solar energy or electricity, you can chemically alter them for a better sheen or new color. It’s only illegal to melt down and re-sell, or re-sell at a price higher than original cost by some arbitrary factor.
Pennies are re-sold at much higher than original cost by huge factors every day: See coin collecting and trading. The mint / government doesn’t mind this at all, in theory because all such coinage is taken out of circulation, thus deflating the economy a small amount, which increases the value of other monies that actually circulate. (See also: Proof and mint sets.) (See also: Stamp collecting, and the fact that the USPS also actively supports this.)
That said, AFAICT, defacing currency is a crime. Counterfeiting is certainly a crime. But I go back to another respondant’s “penny smasher” and railroad-smashing concept: Every government operated toll plaza in Ohio that I’ve ever been to has a penny smasher for the public to use. (It even, -shock&horror-, makes a profit while doing the smashing, as it also consumes 2 or 3 quarters for the privilege of smashing a penny.)
So where is the line drawn? It seems to be like this:
Selling a truck load of copper (or zinc!) pennies as scrap is sure to raise a bunch of eyebrows. Using a handful of them as heatsinks or smashing one as a memento, or using a “Where’s George” stamp on a few C-notes? Not so much.
I’ve used coinage for washers and gaskets and dashboard-quieting wedges and as electrical jumpers, because I happened to have a pocketfull of them at the time and they were suited to the purpose.
Back to the science: The instantaneous heating would be interesting to see, as I’ll bet that the thermal capacity of a penny is greater than the thermal capacity of a paperclip. According to TFS, the paperclip wins long-term, but not all loads are long-term. I also submit that a copper and/or zinc penny will incur less galvanic corrosion in humid environments than a paperclip, and that it’s easier to make a penny mechanically-sound as a heatsink than it is a bit of folded steel wire.
It is legal to damage US currency as long as you don’t try to spend it. It’s a leftover from when currency used precious metals.
Fascinating. So a paperclip works. A little bit of research tells us that they are created from galvanized steel. Not a great heat conductor, but fast enough to wick heat. I’m guessing because of how thin a paperclip is, it quickly saturates. However, it provides the necessary surface area to convect heat out into the air. A new angle would be to paint the paperclip black, and investigate if it also radiates more heat that way, as it increases the emissivity. Several paperclips mounted to the pad, colored with say, sharpie, would probably work about as well as a traditional heat sink.
I’m not sure if “works” is the right word. 90C is hot enough to cause 3rd degree burns almost immediately, and if the case is getting that hot, it’s very possible that the die is exceeding it’s Tjmax
I’ve accidentally caught a 300°C soldering iron by the wrong end and managed to get away with only superficial 2nd degree burns (hurt like a bitch though, not trying that again). I don’t see how 90C would cause 3rd degree burns ‘almost immediately’. That said, I agree with you that 90°C is hotter than you want your regulators to be on a regular basis.
Page 11 of this document: http://www.asse-plumbing.org/WaterHeaterScaldHazards.pdf
shows that you can get a second-degree burn in 1 second from being scalded by 69°C water, so I’m not sure I would want to test it at 21° beyond there!
Yes, but water is wet, and will also conform to the surfaces it comes into contact with. A very hot paperclip or soldering iron will likely only be in contact with your skin for a very short time and over a very small area before your reflexes take you away from the heat, but since water is both conformal and wetting, it makes it much harder to escape from the heat source once your reflexes do kick in. Think of it like cooking, since that’s what your flesh is doing.
I can see a PhD paper in this for someone. “Multiple fractal paperclip geometries applied to the maximal thermal transmission from semocinductor power component sources” i.e. Bent paperclips as heatsinks.
This is inane… not only were the six configurations too close to each other (as pointed out by Leonard), but there was no control! I bet a naked TO-220 would have fared better than any of these.
The only reason the paper clip won was because it was the closest to no “heatsink”. Without airflow, a heatsink is just a big hunk of metal that heats up to the temperature of the package. You need a fan to take advantage of its higher surface area.
This “experiment” suffers from a misunderstanding of the scientific method. Take note.
Yep, no control. Plus tester probably didn’t realize US pennies are no longer copper, unless quite old. Finally, a heatsink made of common aluminum foil will perform better than any of these, and is certainly DIY, but wasn’t tested. Pfft.
It would help if you read the article before commenting. He used older pre-zinc pennies.
“I bet a naked TO-220 would have fared better than any of these.”
I suspect it might have faired as well as any of them, but not sure why it would be “better” the laws aof thermodynamics dont go in to reverse.
“Without airflow, a heatsink is just a big hunk of metal that heats up to the temperature of the package. You need a fan to take advantage of its higher surface area.”
Actually air convection alone will make a difference, a fan will make a bigger difference. My oscilloscope has a whole bunch of transistors on heatsinks, but no fan.
The reason I got it for “very cheap” on ebay was because one of the heatsinks had worked loose and allowed the main switchmode mosfet to overheat. Replaced the mosfet, straigtened up the mosfet heatsink, swapped out a few other bits of collateral damage and it works perfectly. A relatively small heatsink and convection alone is enough to keep the mosfet within its safe operating zone.
You’re right, perhaps not “better”. A paperclip probably increases surface area enough to counter the increase in thermal mass. But without a control there’s no way of knowing how much better (not enough to be worth it, I bet), and I certainly wouldn’t choose to use a paperclip on the strength of a “study” that really only shows how much better it is than giant discs of copper (or zinc).
“relatively small”
Agreed. The pennies are too large.
Seem to recall that copper is better at extracting heat but bad at dispersing it, perhaps a penny with a paperclip star would perform well.
Copper will do a great job at extracting heat, but the surface area for passive heat dispersion is important as well as the separation of the dispersion elements. For passive (convection) heat flow, fin spacing the width of your pinky is about optimal due to the skin effect of the slow air movement. For a this project, I wonder what a copper hookup wire “paper clip” star or flattened scrap refrigerator tubing would do to get the heat out and up with the copper. Then you could cut it like a palm tree to dissipate the heat.
Wow, the quality of comments on HaD really goes down significantly on the weekends. Do you people have horrible day jobs that leave you so bitter during your time off?
I’m shocked at how many people are whining about pennies not being copper, when it says right there in the article that he used older, solid copper pennies.
I personally would have liked to see how a zinc penny would fare against the other combinations…
Pennies 1981 and before are 5% zinc.
In most modern times, that’s correct, but they were made of steel during the second war (we needed the copper to make brass shell casings), and brass just after the second war (we didn’t need the brass anymore).
I’ve also used a small diameter loose coil of 3-4 inches of 12-16 awg solid core wire with the center of the coil soldered to the package. Ended up looking like the curly horns on a Ram. (the large wild sheep) First use got a TO-223 transistor that was running a little hot to run warm to the touch. (~.8 watts load)
I used nickels (thicker, heavier) on a 7805 when I needed it a few years ago. Worked well!
No way does a paperclip have enough surface area to be much use as a heat sink. If the paperclip did end up showing better results, I’d suspect the coupling between semiconductor device and heat sinks, or differences in surrounding conditions.
No way? Apply some geometry and prove it (and don’t forget to show your work), because actual measurements seem counter to your intuition.
I’m thinking that there is close to the same surface area on a paperclip as a penny, and that it is more spread out, allowing convection currents to work more effectively. (And nevermind radiant dissipation, which always works better with a given radiator spread apart than it does with it bunched up, even though the surface area remains exactly the same.)
Ok… I’ll do the math…
number 1 paper clip is 1 3/8″ in length (and about 1/4″ wide) – when coiled
there are three turns, though obviously one loop sits inside another.
At a guess, an un furled paper clip is about 4 inches long. (100mm) and is made of gauge 18 wire. ~1mm diameter.
this suggests that the surface area of a paper clip is (2pi x r x l) 2 x 3.14 x 0.5 x 100 = 314 mm2 (so 3mm around and 100mm long cylinder)
A penny (US) is 19mm in diameter and 1.52mm thick
The surface area of each face is (pi x r) 3.14 x 9.5 = 29.84mm2
And the area around the edge of the penny is (2 x pi x r x h) = 2 x 3.14 x 9.5 x 1.52 = 89.5 mm^2
Total surface area of a penny is therefore 149mm2 (less than half that of a paper-clip)
Not only does a paper-clip have twice the exposed surface of a paper-clip, but a large part of the one of the faces of the penny is not exposed (and not radiating) heat at all as it;s the contact area.
Many people seem to be getting confused:
according to this site
http://www.engineeringtoolbox.com/thermal-conductivity-d_429.html
the thermal conductivity of steel is (at 125deg C – which is close to what this thing was running at) is 51, whilst copper is 400.
So you would think that means that copper is a better heat sink material?
But that’s not strictly true. what it means is that copper is better at conducting heat away from a material, but then it has to conduct something to as well. the metal to air thermal resistance doesn’t change on copper vs steel…
Essentially what this experiment really tells us is that a bigger surface area for dissipating heat is better…
(who’d have thought it, – what with people making heat sinks with fins to hugely increase the surface area for decades.)
and that if your heat sink is very small, then thermal conductance of the heat sink doesn’t matter so much.
so for devices that turn on with a high current very face, wicking away heat fast (so that it’s doesn’t build up at the junction and exceed the max junction temp is important, so copper is good.
For a device that is always on, and needs to dissipate some heat, where the heat source is constant, and won’t peak of spike and need to be wicked away from the junction fast, thermal conductance doesn’t matter so much but surface area of the heat sink is king. -it doesn’t matter so much that the conductance of heat away from the source through the heat sink is slow. it matters that it happens at all.
so to interoperate these results.
>The results were pretty interesting. The paper clip scored better than any of the single pennies! -nope not too interesting, just plain old physics, bigger surface area = better heat sink
>The quad-penny and the Aavid heat sink fared above all the other configurations, – well yes, the quad penny has (almost) twice the surface area of the paper-clip – – loosing some on the faces touching each other. and the heat sink has a huge surface area compared to all.
This is link saying a 2mm copper ball bearing won’t be as an effective heat sink as a steel oil tanker. – or for that matter a brick. – yes I am saying that a house brick would probably be a better heat sink than a very small copper ball, – though I can’t be bothered to prove it with either math or experimentation.
A brick would work as an insulator, meaning it would be worse than no heatsink (or a tiny copper ball, which is equivalent to no heatsink).
Not exactly, bricks make good house materials because they absorb heat during the day and radiate at night, helping keep your house both cool and warm when necessary.
bricks are not insulators in the sense of home insulation regarding heat. – hence why you add insulation between walls
again with the science part:
http://www.engineeringtoolbox.com/thermal-conductivity-d_429.html
thermal conductivity of air 0.02W/mK
thermal conductivity of brick (common building brick) 0.6 – 1 W/mK
I.e. just like a copper heat sink an brick will conduct heat away from a component and provide a large surface area to radiate, and a brick will draw away the heat faster than air. and provide a large surface to radiate heat to the air.
This might be an old thread but….Your math is close for the paper clip (only by sheer luck) but WAY off for the penny.
The proper formula for a cylinder (and both are cylinders) is: 2πrh + 2πr^2 (you have two different formulas and NEITHER is correct.)
So the paper-clip is 2×3.14×0.5×100+2×3.14x(0.5^2) or 314+1.57 = 315.57mm^2.
The penny is 2×3.14×9.5×1.52+2×3.14x(9.5^2) or 90.68+566.77 = 566.77mm^2.
A penny has close to double the surface area. So if they were both in a vacuum, the penny would radiate close to twice the amount of the paper clip if they were made of the same material, same color, etc. In air, the clip MAY have a slight advantage in how the air may flow past it but I doubt it’s really better than the penny.
Now try it again with 1960’s pennies that are made of copper.
Read the article before you comment, please.
No.
Wonder if silver coins would work better since dimes are thinner and quarters are much larger. The change in conductivity isn’t that big between silver and copper (probably less since it’s sterling) but surface area’s for the coins are different.
Also coins do have embossed motives on them, so they are very far from having flat polished surface which is needed to conduct heat well. No heatsink paste will fix this.