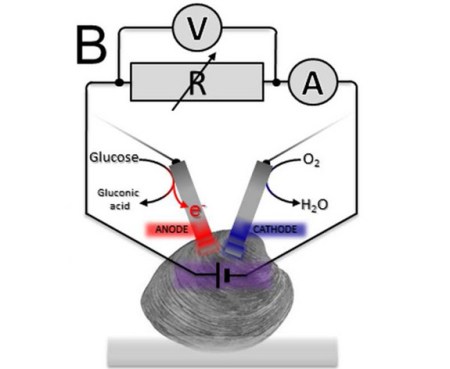
Yes, that is a clam. Yes researchers are using them as batteries. Yes, that quip about the matrix and clam-Neo that is bubbling up into your temporal lobe is appropriate. While keeping a clam as “happy as a clam” might not necessitate a virtual world, they don’t really produce much electricity either. Researchers were able to siphon almost 29 millijoules over the course of an hour. This was enough to turn their electric motor one quarter of a turn.
Wether you find this kind of biological hacking ethical or not, you’ll probably agree that the following quote is, at least a tiny bit, creepy.
The researchers tried different ways to connect three clams at a time as a collective living battery. A serial circuit boosted the battery’s voltage (electric potential), whereas a parallel circuit increased the current (rate of charge flow) — but the overall electricity available often changed depending on each clam’s health.















I’m a real scientist. A tissue engineer. These are not real scientists.
Their conclusion: Clams are bad batteries…
Perhaps I should publish about lots of materials that are bad for tissue engineering.
I recognize the biology with regards to electrical impulses of meaty animals, but what the fuck!
so, compared to a potato power source, which is better?
29mJ over an hour puts an average figure of power at 8uW (microwatts) or 0.000008 Watts.
Wikipedia puts a typical lemon battery at 0.00027 Watts, 33 times more. A potato probably somewhat less.
But still, if blood-glucose could be harnessed as a fuel source, then it’s still a lot more convenient than carrying around lemons or potatoes.
If they could practically harness the power of blood glucose it could go a long way to powering implanted medical devices, how convenient not to be cut open every time your implant needs a battery changed.
@ MGTJ91 – You want this link…
http://www.adameve.com/t-top-10-vibrators.aspx?sc=SEMGLVIB&cm_mmc=GGL-_-Sitelinks+-+Vibrators-_-Vibrators-_-vibrators%20exact&kwid=0503a960e16549d18565960f4c30824f
I wonder if the creatures that hang off the bottom of ships have a significant contribution to electrical corrosion.
Very interesting. I have heard of sacrificial bits of metal on the bottom of ships to reduce the speed of corrosion and have wondered why such things work. Perhaps it is akin to a very low current lightning rod effect.
As far as ethics are concerned it would be better for clams to be useful to the dominant species on the planet. Just think what would happen to the population of sea urchins or cucumbers or Siberian moss if we figured out how to power an iPad with a few of ’em. More interesting questions can be found in a book titled ‘The pig that wants to be eaten’ by Julian Baggini. This ethical dilemma is rather simple by comparison.
that’s another thing, that’s bimetallic corrosion. Metals with different potential in contact with each other can obviously transfer charge between them and thus one is corroded much much more than the other.
It is a problem when you just want to have different alloys in contact for whatever reasin, and it is a “feature” when you use it to sacrifice one of the metals in favor of the other.
http://en.wikipedia.org/wiki/Galvanic_anode
Unfortunately, sacrificial anodes are not used to counter clam electricity, cause that would be cool. Instead the short answer is something along the lines of, the sacrificial material is more easily corroded within the galvanic cell than the material used to construct the ship’s hull.
I guess the clams are lucky they produce more juice when they are healthy and not the other way around. That would make for an unhappy clam.
That makes the assumption that happy==healthy and that is an entirely different debate altogether :)
Been harnessing clam power for more than a decade already. It is more efficient than what they are doing because it gets me going for several hours before it undergoes another process that produces methane :D. Much better than sticking anode and cathode rods on clams. It is more ethical too, I suppose :D
I’m not gonna lie, when I first read the title, vaginas popped into my temporal lobe, not shelled creatures or The Matrix. Bedtime for this guy!
Oh good, this will be perfect to power my crab computer. http://www.technologyreview.com/blog/arxiv/27730/
My computer’s had a virus before but never crabs!
There is microbial fuel cell (MFC) that obtains electron from microorganism (in electron transportation chain) instead of animal.
Sounds more like a power source for nano-machines. One of the major problems in designing virus sized machines that would circulate in your bloodstream and repair the body is a power source!
The human body has glucose and oxygen available to fuel this reaction.
The US military have been doing research for a few years now into generating electricity from glucose and oxygen ions. The idea is to power implanted sensors in soldiers.
I presume this is intended to work towards that, using clams because they don’t move much, and are lower-maintenance than US soldiers. Unless it’s some kid’s science project and he ran out of lemons. Lemon juice goes nice on clams, right?
this is just ridiculous
“…connect three clams at a time…”
Was I the only one that thought clam centipede?
Yeah.
if I just randomly heard that quote out of context while walking around at the airport…
Electric eel.
The Clam Matrix!