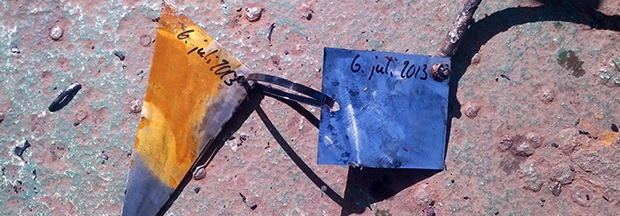
The illutron hackerspace in Copenhagen makes their home on a barge sitting in port. Not only is this awesome, but the members of the hackerspace also worry about corrosion to their beloved fablab. In an effort to ally some fears about rust slowly eating through the hull, [Dzl] has rigged up a cathodic protection system for their hull, essentially preserving their barge at the expense of a few old steel rails.
Cathodic protection systems are able to protect the steel of a ship’s hull by offering up a sacrificial anode made of aluminum or zinc. This can be done by either attaching a sacrificial anode directly to the hull, or with a more complex system that connects both the cathode (the ship) and the anode (an engine block) to a DC power source.
[Dzl] is converting mains voltage down to 12 VDC, then further lowering the voltage with an Arduino-controlled buck converter. The control panel allows for adjustments in the voltage, as well as a nice uptime meter to make sure it’s running.
The results are fairly impressive; in the above pic, the right piece of steel was electrically connected to the barge’s hull, while the left piece was free to rust in the North Sea. That’s only two days worth of corrosion there.















Copenhagen would be in the Baltic, not the North Sea. It’s considerably less saline.
Well… technicall Copenhagen is not in the Baltic sea, but in Øresund, which is the sound (as in body of water) between Denmark and Sweden. To the north of that is Kattegat (between rest of Denmark and Western Sweden), to the south is the “east sea” (Østersøen) which is between Denmark, Germany and Poland.
Baltic sea is way way up north to the east of Sweden.
So, now we have placed Copenhagen right.
Um… need more metal than that. Like: http://image.made-in-china.com/4f0j00lvMakgHCgEWB/Marine-Sacrificial-Anode.jpg
Uh, Adam? That was the test dip they did to see if the system was working. They are using an old car engine block as the anode. Its in the summary.
Don’t any of you guys read the article? You may use an engine block but they use three meters of rail track.
What also helps is they need to save money to get it drydocked regularly so they can scrape barnacles paint the hull. you can not just survive on anti corrosion tactics.
Ultrasonic defouling ;-)
Seems like it would be a good use of some PV panels instead of mains power.
You’d probably need a prohibitively large array to be able to store enough power to run the system though. Average cloud cover in Copenhagen is 63%, and only about 5-6 hours of strong sun in the winter.
http://weatherspark.com/averages/28823/Kastrup-near-Copenhagen-Capital-Region-of-Denmark
What a horrid place to live! They should just set sail to better climes.
Just a small note re: going to drydock, illutron is a nearly 70 year old, 480 ton barge, in Denmark, we’re never going to drydock :) Too heavy, and too expensive. Maybe, one day if we were towed to somewhere in Eastern Europe, we could afford to be lifted out and fixed up in dry dock – but then the towing would cost too much. So, our choice is an anti corrosion system.
Make friends with a diver that likes to tinker. Given enough time they will get under there and spruce things up through their own volition.
Lots of resources go into that type of activity. Better hope this diver has a whole support crew for the dives. and a way to bankroll it.
We actually have divers on our team. They did the hull thickness measurements a couple of years ago. Normally this is done in dry dock but these guys did about 300 measurements under water. :)
This was convenient for me that this came up. My family owns a company that is battling galvanic corrosion on several VERY high dollar green houses that are sitting on lime. The aluminum lexan panel retaining extrusions are turning into a goop that looks like wet silicone. I think the Aluminum itself is acting like a sacrificial anode for the steel support pipes. Not sure if I can get away with driving some sacrificial aluminum anodes all around and giving them some good juice or if I need to go magnesium…
“high” dollar greenhouses… sure ;)
if your relying only on the metal, go with something more reactive than what your trying to save, so Zinc or Magnesium, would be the optimal things. also electrically isolating the greenhouse from steel or other metals would help. if your relying on a – electrical charge you dont really need a sacrificial element.