Like many industrialized countries, in the period after the Second World War the United Kingdom made significant investments in the field of nuclear reactors. British taxpayers paid for reactors for research, the military, and for nuclear power.
Many decades later that early crop of reactors has now largely been decommissioned. Power too cheap to meter turned into multi-billion pound bills for safely coping with the challenges posed by many different types of radioactive waste generated by the dismantling of a nuclear reactor, and as the nuclear industry has made that journey it in turn has spawned a host of research projects based on the products of the decommissioning work.
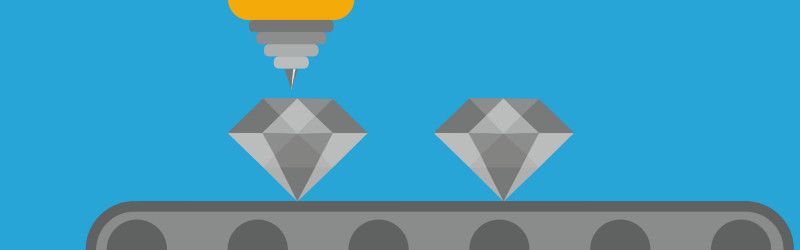
One such project has been presented by a team at Bristol University; their work is on the property of diamonds in generating a small electrical current when exposed to radioactive emissions. Unfortunately their press release and video does not explain the mechanism involved and our Google-fu has failed to deliver, but if we were to hazard a guess we’d ask them questions about whether the radioactivity changes the work function required to release electrons from the diamond, allowing the electricity to be harvested through a contact potential difference. Perhaps our physicist readers can enlighten us in the comments.
So far their prototype uses a nickel-63 source, but they hope to instead take carbon-14 from the huge number of stockpiled graphite blocks from old reactors, and use it to create radioactive diamonds that require no external source. Since the output of the resulting cells will be in proportion to their radioactivity their life will be in the same order of their radioactive half-life. 5730 years for half-capacity in the case of carbon-14.
Of course, it is likely that the yield of electricity will not be high, with tiny voltages and currents this may not represent a free energy miracle. But it will be of considerable interest to the designers of ultra-low-maintenance long-life electronics for science, the space industry, and medical implants.
We’ve put their video below the break. It’s a straightforward explanation of the project, though sadly since it’s aimed at the general public it’s a little short on some of the technical details. Still, it’s one to watch.
Maybe the expense involved limits the number of diamond stories we cover here at Hackaday, but they can still be a hacker’s best friend. How about diamonds in thermal paste, or even for data storage?
[via Phys.org]












We just found the power source for our Proxima Centauri probes.
Even diamonds burn.
Shame the emdrive prototypes are still so inefficient.
Maybe creating a large pile like battery from slithers a few atoms thick then having them fill a capacitor. If the discharge was tuned to the required frequency to drive a resonant coil, it would keep the part count low.
It doesn’t matter that emdrives are inefficient. Check it out Kinetic Energy (KE) increases as the square of velocity (KE=1/2mv^2), but the amount of energy you need from the EmDrive to accelerate is linear (v1=a*t+v0). So at some point, the two graphs intersect and you are gaining more kinetic energy than you are putting in. The same square law applies to angular momentum. All you need to do is get some large wheels attached to a generator with emdrives strapped onto the wheel. As soon as the velocity of the outer portion of the wheel passes the break even point, you can start extracting energy from the system via the generator.
So not only do you not need fuel, you don’t need energy either. Ever again.
There is no, nor will there ever be a, free lunch
Either EmDrive’s claims are false, and there is no free lunch, or the claims are true and there is a free lunch. It doesn’t take advanced physics to get the free lunch either. Breaking conservation of momentum screws things up at a very fundamental level.
The best (and probably only) theory we have on the emdrive, if the tests are correct, is that it loses mass as pairs of photons that cancel each other.
Doubt it as there is barely enough energy for that to happen at gamma ray frequencies/energy
do you have any links, papers etc which suggest it can function at radio frequences such as microwaves ?
The entire claim of the EmDrive is that it gives a greater momentum gain that emitting photons would. If it just emitted photons, it’d be a photon drive. We already understand the physics behind those and can build them.
From Wikipedia Carbon 14 is a β− emitter (electrons). So C-14 is continuously emitting a stream of electrons. The more C-14 atoms you have the higher the current. Since one Ampere is defined as 6.241×10^18 electrons per second, this gives a idea of how much carbon would be required. Also embedding the radioactive diamond inside a diamond outer coating does sounds like the only safe strategy for handling.The battery would approximate an ideal constant current device.
Yes, but they emit those electrons equally on all directions. There usually isn’t a difference of potential, unless you apply some really interesting electrical or magnetic fields into them.
How does just embedding the betta emitters in diamond changes the picture?
You can dope diamond into a semiconductor, make a junction and then ionising radiation will produce a PV like current. So that’s one way it might be done.
The diamond will be at a negative potential (it gives electron) and so be the anode. You just need a cathode (that absorb these electrons) and tadaaaa you have a battery! (Well it’s more complicated than that, I’m not an expert, but it’s the idea)
#skillseroni
I’m presuming that is the case and the BS about radioactive field is BS… Unless theres some further current induced in closely coupled atoms via an electroweak process, collapsing field when beta emitted.
I do have the thought though that diamond encapsulation suggests high embodied energy in manufacture, so these might not be suitable for mainstream uses.
I expect quartz may well exhibit the same electricity polarising properties as diamond.
Diamonds don’t like mixed lattice reflections..
So, charge separation
I hate those ridiculous videos for small kids. Well, I guess a toy invention needs a toy video for an explanation alright
Because its a BS kickstarteresque pitch to gather interest to in turn gather money from investors. They completely lost me when they said diamond = hardest material = best protection… yeah no, it is hard but brittle, drop that sucker and it might shatter into a million breathable radioactive parts… I don’t believe this shit until they show a working prototype.
https://www.youtube.com/watch?v=fkPPkDwH9NA
Ask and ye shall receive- behold! a diamond shattering to millions of pieces.
Why did the diamond shatter the second time but went straight into the steel table the first time?
In other words, why didn’t the point of the diamond embed itself into the head of the press the second time?
Diamond penetrators are the business-end of most hardness testers used on ferrous metals. They work very well and last a long time if they are handled properly however they will shatter if they are loaded asymmetrically at surprisingly low force.
I was thinking slightly more down to earth. Build in an LED and power it from the diamond. Glowing diamond ring!
Had the same thought. Like tritium tubes except better. Although the power source would long outlast the LED
Did you know that if you connect a led to a solar cell and then point the led to the solar cell and give it a small light pulse that pulse will amplify itself untill the whole thing explodes with a bang and a very bright light flash?
no, it wont.
That was a joke, son.
A flag waver.
You’re built too low. The fast ones go over your head.
Smart boy, got a mind like a steel trap – full of mice.
Yes, but amazingly there are people who don’t have the grasp of physics to realize this… and some of them likely read hackaday.
Also if your hand is bigger than your face…
Holy, It is what is wrong with me?!?!?
Few things to consider re this idea/approach & discussion invited especially
for students of Physics/Nucleonics & acquainted with nuclear/electric
generators from thermo-electric to emissive-electric and there are many !
So the issues worthy of addressing are:-
1. Any radionucleotide emission is random in terms of direction, so how
to “vector” the flow still a major hurdle
2. Suggestion diamond will stop/guard/contain beta emissions is very odd
as atoms are mostly empty space & Eg Diamond far Less dense than lead or U238
3. Scalability issues very unclear re C14 especially end to end economics from moderarors !
4. There are far more abundant beta & alpha emitters readily available &
presumably so much cheaper & a greater imperative as taking them out
of an uncontrolled environment to a contained device with recycling regulations
may well reduce background radiation over time.
5. Other, for the enthusiastic to jump in and offer diverse wild speculations ;-)
1 potentially the particular structure of diamond plays some part in vectorising it.
2 electrons not really a point source, it’s less a case of the potential to hit a golfball, through a wide mesh chainlink fence or a number of them. It’s more like you’re hitting a golf ball cored pompom through, where physical drag of the strand would be approximate analogy for electrodynamic drag, leading to higher probability it will be captured by X number of almost empty lattices.
Thanks RW, in response I have:-
1. Hmmm, we talking non radioactive diamond around (ie shielding) the C14 (inside) or
some inherent QM effect which steers as in perhaps “reflecting” beta back to
some conductive acceptor – got a link to a paper on approach, peer reviewed or not ?
2. Sorry none of that gels with what I’ve learned re QM & especially not about point source.
This is all QM based re wave phenomena ie probability stats in the wave (distribution) equations,
in any case tunneling raises its head as well as scattering, absorption & (random) re-emission
re direction and its cascade potential.
Can you pin down any QM aspect which can address this, I’m not getting where you are coming from ?
Eg. A populist source of some QM stuff, good for intro & although a little long
for the general concepts is great for the 1st/2nd year Physics students, maybe at some point
in the series you might be able to address which video in the sequence covers your point re 2 ?
Whole playlist here, can skip most of the into intro stuff if you like & get to the aspect of
orbital interactions with beta radiation re direction, re-emission & transfer of kinetic
energy etc (Think I need to review it as well as has been a while :shrug:) plenty time :-)
https://www.youtube.com/watch?v=Zhq8_63SrvU&list=PL193BC0532FE7B02C
Sometimes you use QM, sometimes you use billiard ball classical mechanics, sometimes you use classical EM theory. In this instance those charged particles are getting acted on by other charged particles. That’s all your getting I don’t do online physics discussion, because I don’t want to end up at bowling ball not falling faster than a BB for the nine millionth time.
Everyone loves Mr Feynman.
Your English is horrible.
– Yours truly, the Grammar Nazi
i’m not a gramma nazi. i’m alt-write.
Well played.
dayam.
Assuming 1/10 of maximum, you would only get 1 watt/ 5 pounds……
but you would only need (slightly less than) half as many kilograms! Think of the savings if we switched to the metric system!
There isn’t enough detail at this point to evaluate this idea propery, and while it may work in theory, there is going to be a long development path that will have to be trod before a practical power source can be fabricated, if indeed one can be at all. They don’t make mention of physical scales and that might be the death of this if these need to be too large to generate practical amounts of power.
Nor am I convinced that enriching the fuel from these old moderators is as easy as the video suggests. In fact if one of the objectives of this project is to reduce the risk from this waste, I submit that processing it for fuel is fraught with far more potential hazard than leaving it be. Not that this would be a reason to kill this battery, but maybe they would be better not trying to spin waste reduction as a benefit.
Time to Build an Iron Man suit screw Arc Reactors
100% efficient? — Ho hum…. So finally a viable battery life for smart watches?
Only if your smart watch uses a minuscule amount of power compared to what we have now, otherwise you’ll be carrying around a kilogram of radioactive diamond on your wrist.
In the article they mention 15 joules per day from a gram, that’s about 17 mW, enough to run a simple watch.
It would help if I moved the decimal point the right way. 0.17 mW, which is still enough for a watch.
A quick google later; “The actual amount of carbon-14 in each battery has yet to be decided but one battery, containing 1g of carbon-14, would deliver 15 Joules per day.” – so something like 0.00017W ?
Sweet, so you’d only need a diamond the size of Rhode Island to power the average neighborhood.
Or a neighborhood’s worth of diamonds spread out amongst the neighborhood. Then an issue with one won’t cause problems for everyone else, unless catastrophic in which case people are already dealing with some major issues.
Someone on reddit figured you’d need a 1m^3 to power a house. My first thought is “damn that’s a lot of storage space to build a building for” until I realized we already have buildings that can store them: houses. Just toss it next to the water heater, or the AC unit, or elsewhere in the basement or attic.The only issue is house fires which would release the C14 if not contained properly.
This might have the methane/methanol problem… it can be undercut by less “green” sources.
Meaning it might be easier/cheaper to irradiate the center of a cheap industrial quality diamond, either mined or mass produced, to turn the middle into C14 than it is to take nuke waste C14 and put a diamond round it.
This work from the guys up at Bristol is more of a capture technology for the carbon 14. As described in the video, just the outer layer of the carbon control rods are highly radioactive, and this can be removed by scabbling (kind of like sanding) or by literally burning the rods.
In the case of burning, either CO or CO2 is created. If CO is created, this can be applied into chemical vapor deposition to create diamond (or graphite or CNT’s, depending on method used). The concept of this work I believe is to condense the radioactive waste of these carbon rods into the smallest possible volume.
The outer diamond uses the same chemical (CO) but with the C12 isotope rather than the C14 Isotope.
As for the shielding and radio-electric (think photo-electric, but using e- rather than photons) properties of diamonds, I’m afraid that is well out of my area of expertise.
I have seen work on using radiation from a material to do chemical work on a system (think making a chemical reaction which requires additional energy).
as for applications? pretty much just deep space / long journey probes. power output will be too low for most other practical applications. solar is plentiful and cheap off grid.
Not just deep space. Also deep water, or anywhere else below the earth’s surface where mainstream renewables aren’t practicable.
C-14 is a Beta emitter, which means even a piece of paper can block the radiation.
Finally, there is a use for Queen´s Jewels !!!
nuclear batteries? i will pass because of one of those explodes radio active exposure is for sure
Radioactive exposure is a certainty your own body contains a stable ratio of carbon 14 from the environment, the basis of carbon dating. Amount, duration, and type of radiation are important in determining harmfulness.
And statistics will tell you that you want to not trust on such ‘harmlessness’.
According to https://en.wikipedia.org/wiki/Linear_no-threshold_model the statistics are not clear for low level exposures, and some even believe that small doses can be beneficial to an organism.
I used the word harmFULness to avoid controversy.
” some even believe that small doses can be beneficial to an organism”
Reminds me of how they used to sell radioactive potions ‘for your health’ back in the early days of the discovery off radioactivity.
I will stick with the simple concept that although your body constantly repairs itself if you get a mutation that is ‘just right’ (or better said ‘just wrong’) you are in trouble. So the less mutations/cellular-damage the better.
Stay away from bananas then.
Whatnot that would mean that areas of the world with higher natural background radiation levels should also show a higher incidence of cancer.
This article https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4674188/ Cancer Mortality Among People Living in Areas With Various Levels of Natural Background Radiation states in the conclusion
“The existing epidemiological and experimental data do not favor low dose-induced detriment but rather agree with low dose being inefficient or inducing benefits by counteracting harm, that is, with the existence of threshold or hormesis.”
It’s a bit tricky to separate areas and assume there is only one factor at play.
For instance you might have an area with lots of radon and another area where there are lots of bananas in the cuisine, and thus the numbers would even out.
Or in another example you might have one area where people are less susceptible to illness due to healthy living and another area with lower radiation. Or yet another theoretical example is that people living in highrises get exposed less to radon seeping from the ground but those same people might travel more often by plane and be exposed to radiation by that means, causing a statistical balancing.
Then there are areas where people smoke more, or areas with coal-powered power etcetera. There are just so many factors.
And I know of plenty of scientist that would advise you that radiation is risky. And even if the risk is low, it’s worse than no risk.
Combine it with the holographic 5D Superman memory crystal and you’d have something like the Kryptonian memory crystal in the 1978 Superman movie.
Isn’t this just a variation on betavoltaic batteries used in pacemakers? We use tritium right now to power them. Clever approach, but I question the manufacturing scalability vs. tritium. Would love to read an actual paper on this if anyone can dig it up.
Okay, let me be the (apparently first?) bona fide physicist to respond to this thread:
1) Betavoltaic batteries are a thing, and are nothing new. See eg this Wikipedia article for a brief overview: https://en.m.wikipedia.org/wiki/Betavoltaic_device
2) Betavoltaic batteries work by effectively the same principle as solar arrays: radiation (of energy larger than the band gap) generates electron-hole pairs in semiconductors. By appropriate doping, you can create an in-built electric field, which will separate the electrons from the holes before they can recombine. They pile up at the ends of the in-built field, generating a macroscopic voltage. Tap this charged region with a contact, put a load between the contacts and presto! Voltage * current = power!
3) Carbon is a semiconductor, so you can do the above with carbon. Diamond is the most convenient crystal modification for that. There are actually radiation detectors built this way for high energy physics experiments!
4) None of the above is new in any way, and also not the point of the Bristol group…
5) Britain has used a lot of Graphite-moderated reactors, apparently. This is not the only way, most reactors these day are decidedly *not* Graphite moderated. Think: Chernobyl…
6) Putting Carbon, mostly C12, into a neutron-rich environment, say the core of a nuclear reactor, makes neutrons stick to the carbon. C12 –> C13 –> C14
7) C14 is a nice beta emitter, so steps 2 and 3 above work elegantly hand in hand. Plus: beta particles (electrons) are easily blocked by even thin layers of matter. Nicely self contained! But still hardly new, see e.g. this patent: http://www.google.com/patents/US9064610
8) *Now* comes the new part: Even for highly radioactive graphite moderator rods, only a tiny fraction of all atoms are C14. Processing tons and tons to extract this is very expensive. Solution: The C14 fraction is highest near the surface, as the neutrons do not penetrate very deep into carbon. Just burn off the outermost layer (radioactive CO2 gas – what could possibly go wrong?), and use that carbon to make diamonds.
All in all: nice idea! It’s bloody difficult these days to get hold of radio-thermal-generators for space application outside Russia and the US, so as a European space buff I say: go for it!!
“Carbon is a semiconductor, so you can do the above with carbon. Diamond is the most convenient crystal modification for that.”
I think what you meant to say was that semiconduction is a property of the lattice and it’s energy levels as a whole, not the element. Graphite is a semimetal, diamond is a wide bandgap semiconductor. So this could not work with graphite for example.
“The C14 fraction is highest near the surface, as the neutrons do not penetrate very deep into carbon.”
I think what you meant to say is that like almost all materials fast neutrons penetrate many meters into graphite and specifically like all good moderators they do this very easily, bounce around a lot until it is sopping wet with epi/thermal neutrons. Being a poor absorber (part of being a good moderator) also means that capture from carbon-12/13 is not normally the majority source of carbon-14.
The reactions that make most of the carbon-14 in a typical reactor are (n,p) from nitrogen-14 and (n,alpha) from oxygen-17, and this is why the the most concentrated accumulation is relatively weakly bonded on the outside of the graphite material. FWIW, I didn’t know this bit offhand, I was just confused by the description in the video and I looked it up.
Diamond based detectors are used in the ATLAS experiment at CERN.
See: http://talent.web.cern.ch/TALENT/research/sensor.shtml
I visited the lab where they built some of the sensors, they got some cool shit. Also, much diamond, very physics.. Wow.
Something similar was suggested back in 1957 — Science Fiction Theater – “The Magic Suitcase”
See it on you tube. Remember watching this back then and thinking that someday it would come true.
i thinl all this is good and well but everyday people ask wil it power my car wil it power something useful for me al the other bla bla is same bla bla as political talk just among them but de 95% of people have no message about that