
A Betavoltaic cell is a device that uses a radioactive source of beta particles and a semiconductor p-n junction to generate electricity. Tritium, an isotope of hydrogen, is often used as the radioactive element. You may think that tritium is hard to obtain or even forbidden, however, recently you can find tritium in self-lightning key chains, and it is also used in watches and firearm night sights. The beta particles (electrons) from the tritium radioactive process causes phosphors in the device to glow, giving a light that can last for years.
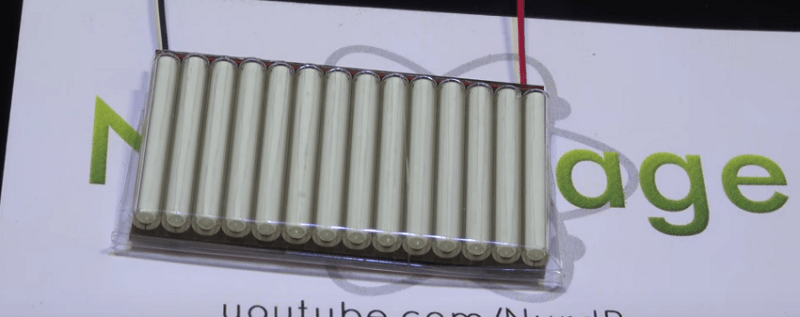
[NurdRage] has just created a nuclear battery using tritium vials from key chains. After getting rid of the plastic containers, he sandwiches the vials between two small solar panels. That’s all! Instant power for the next 15 years. Of course, the amount of power you can get from this device is on the order of microwatts. The battery produces around 1.6 volts at 800 nano amps. He gets 1.23 microwatts, not much, but it is in fact more than the output of commercial units at 0.84 microwatts, for a ten percent of the cost. That minuscule amount of power is actually not easy to measure, and he does a great job explaining the circuit he used to measure the current.
Nuclear batteries in the microwatt range are used in clock circuits of critical computer systems and in some medical implants. They cost approximately $2,200, so you can figure out the price per watt. [NurdRage] version can be built for $220. You may not need one for your next electronics project, but we are sure you will enjoy his project. If you want a light source that lasts for years but do not want to use radioactive tritium, consider the TritiLED.















If he turned the solar panels AWAY from the Tritium, they would produce more power :)
If there weren’t phosphor or glass in the way of the junction maybe, is a solar panel a beta-voltaic in the opposite direction? In this case photons have a little easier path to the photo-voltaic?
I think the joke was that the ambient light is more than the betalights generate.
That’s what I thought too.
everyone knows that beta radiation is a partical and not a photon, right?
The beta particles from tritium decay are blocked by the glass envelope. The only radiation to escape those tubes is electromagnetic, so solar panels are a pretty good choice. Well, tritium decay also emits a neutrino, but good luck capturing that energy.
I thought that it was a *particle*…
Jim, not true, I can detect a tritium tube with my geiger counter. It is weak, but it is there.
@macona: you may be detecting bremsstrahlung x-rays generated when the beta’s hit something in the tube.
Tehe, maybe use the tiny disc from a smoke alarm instead :D
Nope, alpha, too bad.
True statement Miroslav. Best comment so far.
Is the phosphor necessary? Would exposing the bare solar panel to beta rays maybe also result in some power or is the penetration depth too large (probably…)?
I believe that’s exactly what the betavoltaic panels are desisgned to do, as mentioned in the video.
There are nuclear batteries that use the beta radiation (electrons) directly. Some sort of diode-like thing that’s exposed to the isotope directly. But for a hobbyist, handling raw tritium must be a pain in the arse, using the “beta -> light -> solar panel” method is simpler, if less efficient.
Now the next step would be to get a solar panel that was geared specifically toward the light wavelength of the fluid. Good proof of concept though!
Wonder if he could use a filter or lens to shift the wavelength to the ones he has? My bet is it would end up being lower due to the energy cost on the wavelength shift.
Filters or lenses cannot shift the wavelength of light.
Filters don’t shift wavelength , they only attenuate some.
I have always wondered how a solar cell painted with a gamma source and sealed would perform, perhaps it would just wear out like the pre tritium (zinc oxide?) in watch lume while still remaining long term radioactive and still useful were the phosphor not irradiated to death.
I recall an old 80s BBS file or usenet post about painting a beta source into what was very similar to a large electrolytic capacitor and directly harnessing the emitted electrons though loosing their kinetic energy, maybe a mention of alpha emitters in balanced amounts on the other side of a dielectric, the late 80s were 30 years ago. Sounded fun to some kid dreaming of a 10w TEG to trickle charge my bat-cave 160M rig.
Dude, that could have been me… my BBS had 160 megabytes of storage!
Would using less viles together be more efficient? like spreading them across 4 panels
Not by much it would seem, He tried it with one solar panel at first. Adding a second only added ~20% power. The foil reflects light really well back at the panels.
Also mods you really need to move that report button. It’s in the same location as the reply button on reddit for crying out loud!
Or at least provide a popup asking for why you are reporting it, with the option to cancel.
It’s OK, reports go to a human being, who’ll only zap the post if there’s a reason to. There’s hundreds of us complaining a week about false reports, they know. It doesn’t do any harm.
Reddit should change instead.
I’d also test large area photo-diodes for power generation. Unlike solar cells, both leakage current and efficiency are important for photo-diodes so they work well in low light. Surrounding one vial with about 40 BPW34 photo-diodes is likely to produce more power. (You can easily get bigger photo-diodes than the BPW34, but cost rapidly gets out of control) Assuming the same efficiency phosphor, a red or NIR colored tube should put out more power because the photons will match the sensitivity curve of the silicon solar cells better. Similarly, a blue tube surrounded by blue LEDs is also likely to work well.
Thanks! I was thinking of photodiodes but i was unsure about them. I’ll give them a try!
Hey NurdRage – if you’re going to do that, consider getting some 2N3055 power transistors from eBay and cut the metal tops off and try pointing the betas directly at the B-E junction. Much cheaper than diodes.
I do exactly that to make alpha detectors, and alphas are much more easily blocked than betas.
By preference, you will want the older Motorola brand transistors, which have relatively large diode substrates (about a cm square), and not the newer ones which have smaller diodes and maybe passivation layers.
Also, page 6 of this document tells how to calculate the theoretical yield of radiation on diodes.
http://www.ortec-online.com/download/Preamplifier-Introduction.pdf
(And if you’re interested in one already opened and the right type, contact me on .IO and we’ll work something out.)
The 2N3055 is a silicon device. Have you tried this with germanium? Not certain if it would have the proper surface structure – planar – to make it work though..
Maybe selenium rectifier??
Good hack.
If someone removed the ‘glass’ from an EPROM…
You can daisy chain the power and ground pins of EPROMs to make a low output solar cell.
A friend won a prize in a high school science fair doing that with a dozen or so BIOS EPROMs he’d taken from dead and obsolete PC motherboards. IIRC he put 3 or for chains of them in parallel. Might have managed to get a volt out of it.
The nifty bit is the condition of the chip dies doesn’t matter, just that the power and ground are still connected to them. If you can lay hands on a large collection of defective EPROMs…
Honestly, you keep bringing it up!
If I get a chance I’ll try it out. :D
EPROMs are just big silicon die with a bunch of diffused transistors. Do you know if he forward or reverse connected them as the absorption solar cell? I’ve popped open a few older soldered metal top CPUs that are really big die but those might be worth more as collectibles than as science experiments…
This was my idea when I was a kid, except it was with glow sticks instead of tritium tubes. Never got around to developing this because of the half life of a glow stick in comparison…
Nuclear batteries. A staple of science fiction and comic books of the 1950’s & 60s, but not of real life.
An obvious question for a bright kid to think of is, “If there are solar cells that convert light to electricity, and radioactive waste puts out gamma rays for thousands of years, and radiation is just light, then why aren’t there panels to get electricity from that?”
Growing up, I finally got a chance to ask a real nuclear physicist that question, and he immediately answered, “because gamma rays are REALLY hard to stop.”
Apart from all the RTG’s powering, you know, most of NASA’s probes and rovers?
https://solarsystem.nasa.gov/rps/types.cfm
RTGs work on a different principle. Rather than use Gamma rays and converting them directly, they take the brute force approach and stop all the radiation and let them produce heat. They then convert the *heat* itself into electricity using thermoelectric elements (peltier elements). To make stopping the radiation even easier they use Plutonium 238 which releases most of its energy as alpha particles that are easier to stop.
Bottom line: RTGs are NOT gamma ray based. There are no viable gamma ray range photovolatic panels due to their extreme penetration depth. It is more viable and compact to use the heat from the decay instead.
…and lighthouses:
http://gizmodo.com/5132765/soviet-atomic-lighthouses-are-both-spooky-and-deadly/
An object lesson in why nuclear power, though safe, clean and cheap, needs to be properly and responsibly managed throughout its ENTIRE life cycle (whichis longer than yours by several orders of magnitude).
We don’t worry about the half life of the dangerous radioactive elements in the Earth’s crust, we just sit secure in the absolute fact that they are not going anywhere. If you simply bury radioactive wastes where nobody can get to them, the problem is solved.
I expected NurdRage to use the tritium to hydrogenate an oil then coat the semiconductor cell with that directly. Probably a little dangerous but it could be polymerised to make it stable then you could seal the assembly in a glass tube with the ends heated and closed off like a valve or light bulb.
You tried doing that and the DOE/NRC will be all over you. Doing anything with tritium gas other than it’s intended product is a big no-no. Technically it is illegal to even take apart a tritium exit sign to remove the tubes.
Those tritium keychain lights aren’t even legal in the US, technically.
(But of course if you need a hundred tritium gunsights well that’s just fine, for freedom.)
Those exit signs can be a total of 20 curies in each sign… quite a lot.
The battery might work better if some of those were obtainable rather than the tiny ones.
He used the multimeter wrong. When you switch from volts to amps you need to use a different port on the meter… lol cmon nurd rage
most of my multimeters will read a couple hundred milliamps without having to switch over to the larger current shunt port
Same here, milliamp range is read on the voltage input.
same here,
people who can NOT make accurate mA readings are usually the same people with this setup… it doesnt take long to shift the value of the current-sense reistor.
unplug;safety-first
(then change function)
you could ballpark-it by comparing it to the Amps range ie 172mA vs 0.16A miiight be around 166mA, or just permenanently solder tweak-resistors while borrowing someone else’s meter to compare.
The DVM I use most reads up to 2A before shunt needed.
Many years ago I had the idea of painting a solar cell with luminescent radium watch dial paint. I wondered how much power that would put out. Never tried it since apparently you can’t get radium paint anymore. :) Then when I watched NurdRage’s video on the Soxhlet Extractor where he was removing the plastic shell from the tritium key chains I thought “Those would be perfect for my old idea of a nuclear/solar cell battery. Didn’t even occur to me that was already where he was going with that. :)
I had considered that as well, sandwiching the source with two solar cells. But back in the day we only got about 10% efficiency.Plus I was lazy, so I dropped the idea.
Please, please, please, send your battery to Dave Jones of EEVBlog.com. I’m sure he can reproduce and improve your measurements, make another nice youtube-movie about it, and even perhaps come up with an energy-harvest solution to put it to good use.
You’re welcome!
Those vials can be bought separately on ebay. I bought one and 3Dprinted a parabolic case over it.
Hmm, so 1.23 µW over 15 years equals 0.16 Wh. Its a cool hack, but I wonder in what case would it be better than just a simple lithium primary cell like CR123?
Oh absolutely. This is an utterly useless device for almost every purpose. It’s only the extreme cases where this might be viable.
Like if you need something that can work at -100 celsius. At temperatures that low most batteries freeze and some will even puncture their internal separators, causing them to short out if you bring them back up to room temperature.
Other cases include needing absolute 99.9999+% reliability. A chemical battery SHOULD last 10 years. But you might have gotten the bad batch that lasts 6 years. You won’t know until you wait that long and try it. A nuclear battery is extremely predictable. I don’t have the specific numbers myself, but you can predict to within a few percent the power output at any point in the 50+ year future by taking into account the decay rates of the nuclear material, the phosphor and the solar cell. Knowing that, you can build with extra capacity and be extremely certain you’ll still have enough power at some far off point in the future.
But you are right, for most people, even most professionals, a bunch of ultra-long life lithium batteries with some ORing diodes will give plenty of capacity and reliability for most projects which only need to last a few years at most. If you’re really building a 20+ year device. you wouldn’t be hacking a nuclear battery yourself and you’d buy a proper one. This is just a fun project for those that want to add “Made nuclear battery” or “Built nuclear powered X” to their achievement list.
Enjoyed your article. I am writing a manual explaining some of the future technologies in my novel, “Texting and Teleporting”. One technology is a nuclear battery. I am an engineer with a nuclear background and considered a design that can deliver 50 watts. The mention of 20 curies in a fluorescing exit sign made me think twice.
I think to install atomic battery for my electric DC motor
I’ve been looking into building a battery/solar generator lately, and I was wondering how much tritium would be needed for the battery and if it would be feasible/possible to create a nuclear battery that large, say a 12volt battery powerful enough to drive a 1200 watt inverter? 600watt? D
No, it wouldn’t, it’d be massively impractical. His only puts out about a microwatt. Build a million of them and you’ll have 1 watt. So you’d need 600 million little solar cells with however-many billion of those tritium tubes, which will conceivably cost more money than there is on Earth, probably.
You’d be better off nicking an RTG from an old Soviet lighthouse, or perhaps a forgotten space probe. Or get someone to nick a space probe before NASA get a chance to launch it.
Or else build your own, it’s only a KG or two of plutonium you’ll need, and the facilities to not die while you’re working with it.
Probably best to point solar panels at the actual Sun. A nice safe fusion generator that’s thousands of miles away.
I realize this is an old discussion but it occurs to me you could possibly see *some* gain by encasing the whole thing in clear epoxy (vibrating out any bubbles) to transmit the light more directly to the PV cells. Just a thought.
Epoxy surely blocks light more than air does. And there’s nowhere else for the light to go, than the solar cells.
You should of used blue….. the spectrum emits more photons.
I used a kids plastic mirror to reflect the light that’s going out and not into the solar cell, a big improvement bit still i don’t understand the wavelengths and all the other stuff still learning
Hey guys,
I found my way to this article as I work in a laboratory that deals with 20-50Ci iridium-192 isotopes housed in depleted uranium containers. I wanted to make an arduino GPS logger to attach to each container to automatically log the source movement (rather than the current paper method) as it’s, understandably required by law.
The schematics i’ve found for GPS loggers only have a limited battery life, so I was wondering if the radiation from the isotope could be used as a power source to aid and recharge the battery. You get about 100mSv of radiation at the edge of the container.
Would this be possible?
stolen my idea. ???? anyway with a dc to ac power inverter and a Walton cockcroft voltage multiplier it might be possible to power a light bulb or a small radio ???? ????????
Also worth trying: get some ancient photoelectric smoke alarms and pull the IR sensitive photodiode out of them.
They are quite good at converting infrared to low voltage and the ones I found are maybe 0.8V >1mA under moderate flux.
Maybe a better bet would be to get red trasers not green as the peak wavelength is closer to the peak on solar cells?
Also be careful with epoxy, it can crack the glass!!
Maybe safer to use nail varnish that sets under blue light.
What if you took tritium paint and painted it onto the solar cell(s). They are really just arrays of P-N junctions right?
Maybe I should read the article before commenting… :-)
More power from a lemon cell. Pump that up a bit and put that into a real battery cell. This photocell idea is not a ‘battery’.
id say make a phosphorus silicone composite to replace the tritium casing / glass and the monocrystalline layer on the panel attach everything directly
So basically the tritium emits enough light to the solar panel to generate movement of electrons and therefore creates current. Is this what I am getting? Because of the low emission of light the device can only output small amounts of current. Therefore to obtain said materials prohibits mass production because the light emitted off the tritium is so small and the capacity of power generation is so large for the panel that cost vs. efficiency is not viable.