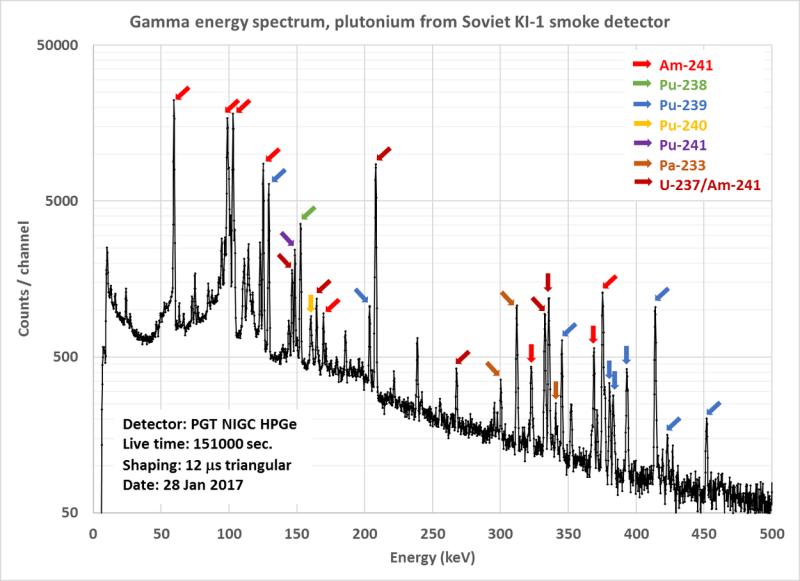
It’s widely known that a smoke detector is a good ionizing radiation source, as they contain a small amount of americium-241, a side product of nuclear reactors. But what about other sources? [Carl Willis] got hold of an old Soviet era smoke detector and decided to tear it down and see what was inside. This, as he found out, isn’t something you should do lightly, as the one he used ended up containing an interesting mix of radioactive materials, including small amounts of plutonium-239, uranium-237, neptunium-237 and a selection of others. In true hacker fashion, he detected these with a gamma ray spectroscope he has in his spare bedroom, shielded from other sources with lead bricks and copper and tin sheets.

He digs further into this analysis, using the ratio of plutonium to americium to determine the age of the source, concluding that the radioactive material in his sensor was produced in 1968, then processed in about 1972. By digging into the statistics of the ratios, he concludes that it qualifies as reactor grade plutonium, not the purer weapons grade that those looking to make big booms seek. It is also worth pointing out that we aren’t talking about enough plutonium here to do much with: he estimates that there is less than 1 mg of plutonium-240 in the source. It’s also worth remembering that the americium in your smoke detector came from plutonium: it’s a natural decay product of it, although the stuff used in the US has the plutonium removed. We’ve featured the work of [Carl Willis] before, such as his home-made Farnsworth Fusor that can fuse hydrogen into helium.















It stands to reason. Using americium in a Russian smoke detector would have been considered unpatriotic.
“The high-energy gamma ray detector that Carl Willis used to analyze the soviet smoke detector, located in his spare bedroom.”
:O :D
This is the guest bedroom, “the least radioactive room, as it should be”. Such consideration for guest! Guest: “What’s that behind the lead shielding blocks?”
Sounds like something out of XKCD…
Don’t touch anything behind the giant warning sign, you’ll ruin the experiment!
For awhile they were using Pu powered RTGs for implanted pacemakers here in the West. In these too the amount of material was very small, but upon the death of the user, the country’s nuclear regulator had to be called to recover the unit and see to its disposal.
Can confirm.
https://www.orau.org/ptp/collection/Miscellaneous/pacema3.jpg
I would be sure a favorite geek grandkid knew where there was a razor blade for my ultra rare final gift. Several hacker lifetimes of beta heat output for a small TEG circuit from that pellet, and safe as long as nobody breaks the pellet’s capsule.
alpha
If he’s willing to cut his recently deceased grandfather’s corpse open with a razor blade, he’s not the sort of kid I’d consider my favourite.
What’s the output of those pacemaker power units anyway? Anyone know?
I suppose you could always arrange your funeral with an undertaker who has a secret. A good 80% of them are fucking the corpses, so blackmail one! Tell him little Jimmy gets your pacemaker, clean and shiny, or else a trusted friend releases the tapes.
This thread went down a very dark path!
As I recall the Soviets developed Atomic batteries for remote beacons. Atomic batteries are not RTGs but use beta particles striking a semiconductor junction to generate power. Turns out not all of them are accounted for. I recall a story about a hunting party a number of years ago that got a hold of one in a wilderness area and got very sick.
Most of those beacons were powered were powered by RTGs it was the pacemakers that used betavoltaic devices. The Soviet units used Strontium-90 as a source which is indeed mostly a β emitter but the device produced electricity by thermal conversion.
They also used them for their nuclear lighthouses which their submarines were very dependent on for navigation. (see comments)
http://englishrussia.com/2009/01/06/abandoned-russian-polar-nuclear-lighthouses/
According to the World Nuclear Association, “An average smoke detector for domestic use contains about 0.29 micrograms of Am-241 (in the form of americium dioxide), so its activity is around 37,000 Bq (or about 1 µCi).”
Modern smoke detectors, yes. I have a couple from 1990 that are labeled at 0.9 uCi each. Older detectors (1970’s?) used more.
Yeah, apparently the 1970s ones can have as much as 80 uCi of activity and a lovely tendency to leak: http://www.diyphysics.com/2013/01/20/80-%C2%B5ci-americium-241-sources-inside-old-pyrotronics-f35a-smoke-detectors/ (Link found in this post.)
In my area they use optical ones without any radioactive elements, and they work fine.
I really see no need for anything else in a normal domestic environment.
Especially since the majority of the smoke detectors in operation in homes have flat batteries I’m told.
While that may sound like a lot, a Becquerel is 1 particle per second, so the radioactive source in a smoke detector emits only 37,000 particles per second.
That’s in all directions, so from any individual direction you won’t get that many particles regardless of the particle type, and exposure goes down by the inverse square law.
It’s not uncommon to see 20,000 cps samples (of various ores) for sale on eBay, and that 20,000 is measured by a geiger counter with a tiny aperture relative to the surface of the sample, so those samples have a whole lot more Becquerels in radiation that’s a whole lot more dangerous than Alpha particles.
United Nuclear sells whichever samples they have, they have one at about 10,000 cps right now, and I remember they once had a sample that they claimed measured 140,000 cps.
All this hype about the dangers of smoke detectors is completely overblown.
“Ionization smoke detectors containing less than 1 μCi of Americium-241 are exempt from Nu-
clear Regulatory Commission regulations. This means that Federal Law does not prohibit the
disposal of these detectors in the normal municipal waste stream. There are, however, a number of State and local regulations and/or laws that do prohibit disposal of ionization smoke detectors in the municipal waste stream. Contact the local solid waste management authority for up-to-date information about local regulations or directives.
Older ionization detectors that contain more than 1 μCi of Americium-241 are subject to regulation by the NRC, and they are subject to more stringent requirements. Smoke detectors with 5 μCi or more of Americium-241 should never be disposed of in the municipal waste stream.”
——————
So why not make a phone call and dispose of properly? Everybody happy.
Had Doc Brown only known…
lol
Would have to steal a few hundreds smoke detectors to make one time jump.
I wish he had written more about the instrument setup used for the measurements, but Claire Stong, the Amateur Scientist columnist, would have loved the post anyway. Fabulous work.
If you go back through Carl’s other posts there is a wealth of other good content covering gamma and alpha spectroscopy, neutron statistics, nuclear forensics, the care and feeding of HPGe preamps, and a wealth of other good stuff.
Here are a few nice posts. But seriously just go read every single one on his blog.
https://carlwillis.wordpress.com/2016/06/04/herb-andersons-live-block/
https://carlwillis.wordpress.com/2011/11/04/gamma-activity-measurements-of-tokyo-area-soil-samples/
https://carlwillis.wordpress.com/2012/08/19/gamma-analysis-of-chagan-atomsite/
https://carlwillis.wordpress.com/2009/09/18/refining-uranium-by-the-purex-process/
https://carlwillis.wordpress.com/2011/09/16/hpge-detector-part-i-repair/
Carl is the real deal, but I really hoped when I read the headline that it wasn’t Carl. He’s the only person doing this at this level. He’s the Ben Krasnow of home nuclear physics. Last I remember he had a chunk of the original Chicago Pile in his spare bedroom, so I guess he’s got a fair few gammas to shield his detector from.
Best way to keep the in-laws from coming to visit. Sure you can stay the night, just move the radioactive material off the bed first…
Carl’s justification for using the guest bedroom for the gamma spec is that it’s “the least radioactive room in the house”. :)
Uh, i hope this guy knows what he is doing. :-/
Considering the lethal dose for plutonium is between 1ug and 20mg (depending on the scientific source): 1 mg is already a lot !
Well it depends on how it would get into the body. A solid chunk is not going to be as much of a hazard if is handled with common sense. Grind it into a fine powder and run it up your nose, and you would be in a bit of trouble.
1ug? That’s a bit of a fiction.
Many people, particularly people doing early work with large amounts of plutonium at Los Alamos for example, have ingested or inhaled or impaled detectable amounts of plutonium, and have lived normal lifespans, carrying a detectable body burden for the rest of their lives.
Ah the good ol UPPU party. With a total of 26 members, the “You Pee Plutonium” was pretty exclusive.
Need to know more! Reference?
I thought it was IPPU
Ever since I watch Carl’s YouTube videos I can only read this article hearing his southern drawl in my mind. It’s almost like Sheldon Cooper from that horrid TV show.
Speaking of his YouTube channel he and bionerd23 do a few tours of Chernobyl’s reactor halls and find some nice hot particles on the side of the road.
“I made an attenuator out of rolled cadmium sheet and endcaps stuffed inside of a piece of copper water pipe with copper endcaps. Such an arrangement works by strategically situating the K-edge energy of the absorber materials close to the energy of the offending radiation.”
Ah yes… that hoary old chestnut.
“…shielded from other sources with lead bricks and copper and tin sheets.”
Welcome to another episode of Fallout 4.
A “lead castle” is an important part of any gamma spectroscopy setup, even professional ones ;-)
There is nothing unusual about this. Americium 241 is transmuted from Pu239 by two successive neutron captures, and Pu241 beta decays into Am241. It’s not uncommon for ionization type detectors of that era to have impure sources due to the difficulty of isotopic separation. I have some American made sensors from the late 50’s with similar isotopic composition.
But this example is roughly a decade and a half newer.
It worked. That’s the metric used in Russia.
EXACTLY. Damn CLICKBAIT!
Is it save to use smoke detector or gas/CO detector as spare parts source for diy? For instance power supply with very low consumption or loud alarm circuitry?
Looks like something to do in a classroom. I was rather thinking about using electronics spare parts for “conventional” diy projects. For instance sensor’s loud buzzer/speaker for alarm system.
Any experience with that? Safety concerns?
Remember the Nuclear Boy Scout (David Hahn)? He tried to build a atomic reactor in hist garden with smoke detectors.
Where can you buy lead bricks?
They are not bricks per se, but lead ingots. One of the standard sizes is (was?) the about the same dimensions as a brick.
I think the confusion here is this line: “high-energy gamma ray detector”. I’m surprised more people here didn’t recognize this as a *LOW* energy and *LOW* intensity gamma spectrometer setup. The lead bricks aren’t there to shield you from the sample, but rather the detector from YOU (or background radiation, generally).
For an extreme example of this, read this article (and Google others): https://www.scientificamerican.com/article/ancient-roman-lead-melted-down-explore-frontiers-physics They want this lead because it contains a very low amount of Pb210 which is radioactive (Pb206 is the stable one), while ordinary lead contains much higher amounts of Pb210 as it’s part of the U238 decay series (along with Ra226, Rn222 (household radon), and Po210).
As a parallel example, he (and the many other labs doing this routinely) is trying to create the radiological equivalent of the National Radio Quiet Zone (NRAO) for radio telescopes: you have to kill the background noise to “hear” the signal you want to pull out.
The lead story is indeed true.
Incidentally this is (rumored) to be why a particular type of Geiger tube sells for ridiculous prices as the special lead alloy used in connecting pins and shielding is “low background” and harvesting it carefully won’t generally damage the tube so it can be reused.
Also feasible: harvest it from dead light bulbs made by manufacturers that no longer exist.
Is this is purely down to the length of time since the lead was smelted, then UK churches and cathedrals could make a pretty penny by replacing their old lead roofs, and selling off the old lead sheeting.