In his continuing bid to have his YouTube channel demonetized, [Cody] has decided to share how he makes chlorine gas in his lab. Because nothing could go wrong with something that uses five pounds of liquid mercury and electricity to make chlorine, hydrogen, and lye.
We’ll be the first to admit that we don’t fully understand how the Chlorine Machine works. The electrochemistry end of it is pretty straightforward – it uses electrolysis to liberate the chlorine from a brine solution. One side of the electrochemical cell generates chlorine, and one side gives off hydrogen as a byproduct. We even get the purpose of the mercury cathode, which captures the sodium metal as an amalgam. What baffles us is how [Cody] is pumping the five pounds of mercury between the two halves of the cell. Moving such a dense liquid would seem challenging, and after toying with more traditional approaches like a peristaltic pump, [Cody] leveraged the conductivity of mercury to pump it using a couple of neodymium magnets. He doesn’t really explain the idea other than describing it as a “rail-gun for mercury,” but it appears to work well enough to gently circulate the mercury. Check out the video below for the build, which was able to produce enough chlorine to dissolve gold and to bleach cloth.
We need to offer the usual warnings about how playing with corrosive, reactive, and toxic materials is probably not for everyone. His past videos, from turning urine into gunpowder to mining platinum from the side of the road, show that [Cody] is clearly very knowledgeable in the ways of chemistry and that he takes to proper precautions. So if you’ve got a jug of mercury and you want to try this out, just be careful.

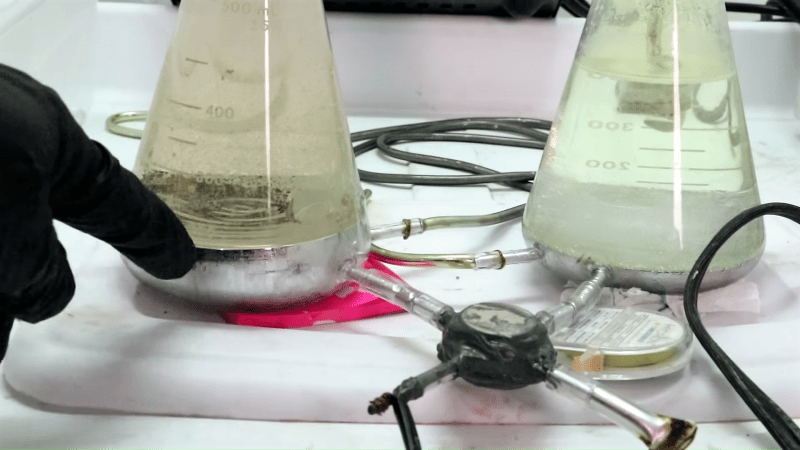














Pump is probably using principles from magnetohydrodyamics.
https://en.wikipedia.org/wiki/Magnetohydrodynamic_drive
Apply magnetic field in X direction, apply electric current in Y direction, force is developed in the Z direction. If the thing carrying the current can move, it will. If that thing is a liquid, you have a pump.
Another version is just to spin the magnet around, because the induced current will make the metal act like a magnetic brake and drag it along.
Yes, but a pump with no moving parts except the fluid being pumped, has a coolness multiplier of about 5.
Captain Ramius would agree.
Tech Ingredients posted a video about this recently:
https://www.youtube.com/watch?v=LS3GQk9ETRU
Here’s the video about the liquid sodium-potassium fountain the University of Nottingham made using the technique:
https://www.youtube.com/watch?v=9EGAXOWpGy8
https://www.youtube.com/watch?v=9EGAXOWpGy8
It’s this pump: https://www.youtube.com/watch?v=9EGAXOWpGy8&user=periodicvideos
just working sideways rather than vertically, thus avoiding issues with how heavy mercury is.
The first dude in that video reminds me a bit of Spinal Tap’s manager, Ian Faith
https://youtu.be/S3nuI4oEal0
Interesting, wonder if you could sit salt on top of mercury and applied associated terminals in say gentle argon flood, I guess heating the mercury isn’t healthy or the chlorine emissions.
Reason I think that at first is it reminds me of high school days electrolysis of sodium nitrite heated to just on melting point in a steel dish (Bunsen underneath back then 50 yrs back) whilst incandescent bulb very gently placed into liquid NaNO2. Negative terminal on bulb with positive terminal on steel dish with variable voltage current limited supply. Turns out the sodium migrates gradually through the glass when hot enough but not too hot to melt the glass to coat the inside of the incandescent bulb so you can turn a clear bulb into a localised reflector at whatever angle you wanted or make other oddities a strip at a time. Be careful hot liquid NaNO2 dangerous and slow application of heat to bulb and of course in time hood behind shield and glasses too.
*fume hood not time hood, lol though close…
Awesome, never heard about that before! Yeah, that is a new method to mirror glass that seems interesting. OK… calming down.
I think it goes back to the 1930’s or so, can also use other alkaline salts that have lower melting points than the glass, minor complications with Pyrex and borosilicate re migration times. The amount of time it’s active for the sodium or potassium to migrate means very little if any gaseous evolution other side eg not much NO2 etc. Potassium interesting as it’s transparent to some UV spectra but reflects visible. Can have some very interesting optical effects mixing both sodium and potassium alternate side of a suitable mercury vapour lamp and illuminate with appropriate UV light source and fluorescent materials. Have a few of those foot long mercury vapour lamps left before I normally cut em up for large test tubes…
I think I want to avoid working with materials that migrate through glass.
I think mercury can migrate through plastic (or some plastics) I want to say.
I find it pretty hard to imagine a metal migrating through plastic. Sure you aren’t thinking of mercury compounds? Maybe dimethylmercury?
I recall more hear-say from my Chemistry colleagues over the years.
Here are some references off the net:
https://water.usgs.gov/admin/memo/policy/wrdpolicy82.112.html
https://chemistry.stackexchange.com/questions/80511/why-are-plastics-lipophilic
https://ecologycenter.org/factsheets/adverse-health-effects-of-plastics/
https://books.google.com/books?id=HXXxw2djkcsC&pg=PA243&lpg=PA243&dq=mercury+diffusion+into+plastics&source=bl&ots=p4UyDHh3Xm&sig=kbQnrnuunBIZ-CFFqwG_mJHyR3U&hl=en&sa=X&ei=BhD9U4fSGcipyATE6ILIAw#v=onepage&q=mercury%20diffusion%20into%20plastics&f=false
Personally, rather than combine the sodium with water to make NaOH, I’d want to keep the sodium, it’s more fun to play with (and harder to just buy). Chlorine has its uses…now and then…but at least in my life is more useful in compounds that keep it out of my respiratory system. Or, as Cody says, “blech”.
But, cool beans like most of what he does, and the MH pump is super nifty – you don’t get many chances to use one, since most other liquids aren’t that conductive and/or would electrolyze themselves.
Battery acid + sea water would be my approach.
Oh yes, every submariner’s 3’rd biggest fear after drowning and fire.
“Battery acid + sea water would be my approach.”
Does not produce chlorine.
If the mercury in the cell is not circulated the risk is that at fairly low amount of sodium it sets solid.
That’s only a issue if you drive a Tesla or own a sub. It’s really funny that Teslas and subs have the same problem.
Cody suffered previous take downs. The first was for making Thermite, Cyanide or similar. Apparantly Youtube thinks they are a television network liable for Viewers taking his videos literally?? Second time it was a Flat Earth conspiracy flagging his videos an mass until he was blocked. That was cleared up in a few weeks. Flat Earthers were using clips from his videos to prove rockets didn’t work in a vacuum (through heavy editing) So Cody made a polite video response stating how these flat earthers are outside functional thinking. Making Chlorine on You tube however is not the smartest thing to do if you wan’t to keep your channel up. Chlorine have been used historically by crackpot organisations to get attention.
Perhaps, but it is at best delusional to suggest that crackpot organizations need help from YouTubers to do thinks like make chlorine.
Common sense isn’t very common. Cody is waving a red flag in front of Youtube to ban him. But then again I half expect Cody to be found dead or end up killing someone else with Chlorine gas who tries to replicate his work in their bedroom or kitchen table
BTW Chlorine gas is nasty stuff – it will shut down your respiratory system in seconds.
Interesting and even more-so how mercury has been used in the world of electrochemistry… of which… isn’t usually as clearly taught from my experience, though has an older history than most realize from what I found in my brief research. I had a book from the 1800’s that I’ll try to find as I’m not finding online now and forget the details. Was interesting.
I keep thinking it was one of these books… though know it was a broader reference book not only related to gases. http://knowledgepublications.com/hydrogen/hydrogen_energy.htm
Thanks jafinch78,
I like seeing more publications around investigating ‘out there’ approaches especially that might just have some pragmatic connection with well articulated experimental methods.
Some 20+ yrs back I read a post by iirc Bob Lancaster who indicated his end to end thermodynamic analysis in respect of hydrogen in terms of generation and utility showed it was a net energy loss – well that is based on the exploitation tech (from fuel cells to engines etc) of the time regardless if the origin from water had 100% electrolysis efficiency :/
In effect Bob inferred and with good engineering authority, if you like, that if the source of hydrogen was completely free of cost it would still be too expensive to use – likely in relation to the tech of the time in respect of flow, loss, storage, controls and in conjunction with net energy output including its effect when used on engines/vehicles and its displacement of more energy dense storage methods than as a liquid when chemically bound ie Petrol.
It’s worth considering there is more hydrogen in a litre of petrol than an appropriately chilled litre of liquid hydrogen and since H2 liquid inappropriate to even try expend even more energy to compress ie. Pointless.
Makes you wonder if any energy system’s explorers even understand what an ‘end to end’ thermodynamic calculation actually means and what extraneous factors it really needs to encompass (net present costing methods too) in terms of most practical comparison with any other options for common transport. Perhaps in purely stationary considerations it might be more viable but, as I recall even in that situation it’s still a net energy/$ cost overall with tech of 20 yrs ago, so has that changed much – I think not ? So any major co or government moves to H2 for transport either PR or outright ignorance as I cannot as yet see the incremental change in tech offering any differential advantage from Bob’s deduction back then.
Bob also added iirc that any free source of electricity totally wasted on hydrogen, far better to produce aluminium or titanium etc such as more utility, commodity, greater ease of transport etc… The only area H2 was worth anything in bulk is launching for NEO craft. Smaller qty’s of course for Chem but, that’s all :-(
I recall BP in Perth Western Australia routinely flare off H2 from their catalytic towers and when the hydrogen bus trial occurred some 15 years ago they rubbed their hands in glee charging heaps to collect it ! Iirc the buses only went 80Km on a fill and back then a mech engineer worked out it would be cheaper to feed the buses with a fresh replacement of D sized alkaline cells each time for that amount of electrical energy for distance travelled not even worrying about amortizing the setup of AUD$900,000+ cost of the two buses and not even including maintenance such as refurbishing the fuel cell electrodes. To this day I see very little has changed about utility of H2.
Yeah, I didn’t get into the calculations so much. My thought was more on the line of excess energy to be used in off the grid situations, or maybe nuclear plants, instead of creating heat in a resistor… maybe even on a homestead storing as a gas that can be later used if only to help catalyze the combustion process to make a cleaner burn or use of more types of fuels. In regards to storage.. yeah… there needs to be some sort of reaction, or binding of, the hydrogen to decrease the volume and increase the density. I think the main point I took is cleaner and higher efficiency combustion processes using a range of fuels and not hydrogen primarily for now. Might be some sort of future innovation potential or need.
FWIW There are other issues with hydrogen not well touched on and one might interpret sidestepped quite a bit which has to be dealt with in terms of infrastructure costs hence the analysis and risk assessment, such as:-
– H2 tiny so tends to leak out all too easily
– More powerful than CO2 as green house gas
– Embrittles many metals, for a meyal pressure container makes it dangerous, others expensive
– Adsorbs to various surfaces and with a layer too causing hidden fire risk
– Very easy self ignition. Eg open valve on H2 tank, can ignite by its own flow electrostatics
– H2 fire mostly invisible very hard to manage safely, see fire management guidelines
– For engines, energy density very low displaces air
– Not completely clean burn, some NOx and HC’s (from chamber lube eg walls)
Very keen to find contemporary full on ‘end to end’ thermodynamic analyses. The recent push for H2 for transport fuels just doesn’t make sense in all sorts of ways unless there are very good tech reasons and so far the bulk of those are no where near mature and still rather expensive.
FWIW. In my considered engineering opinion:
The best solution to any excess H2 would be reforming alcohol based liquid fuels at location of best utility such as butanol and other oxygenated fuels and up into higher order basic ones to start with ie. more dense liquids with higher specific energy per Kg as transition to electric transport displacing internal combustion engines in short to medium term. The way battery and super cap tech is going that would be the least troublesome transition method by far not shifting to H# for any bulk purposes. Any pollution issues with current liquid fuels such as NOx addressable in that transition with better active catalytic converters in any case short term. We already have sizable liquid fuel infrastructure globally so not efficient to sidestep it yet for H2 only need comparatively minor adaptations, use it in transition to overall electric based…
I’m really sorry, but what is the blog your name links to about?
No need to be really sorry or apologize. I’m not sure what is linking… dewdefenseprojects.blogspot.com or dewdetectionprojects.blogspot.com. (Writing my thoughts down).
After clicking on what my name links to… you reminded me I started a WordPress page and never worked on it after setting it up. I think I got a few hours in and that’s it.
Man, thanks for the reminder.
The WordPress link is about the blogger blogspot stuff you can read about in more detail on the first post from August 2017 regarding Cuban Embassy and other Embassy or local issues like reported before in Moscow, Uzbekistan, Israel/Palistine, China half arse and elsewhere that happened to me since the Clinton Administration from what I can remember and always horrific during U.S. Democrat Administrations.
I get sound malicious assaulted with intent to destroy personality, property, person and people groups, communities, economies, society and the peace all the time from armed and unlawful alien/enemy combatants and sometimes body assaulted now days with some mind control and more mind control from whomever is my controller and/.or handler since I complained a bunch and I guess was a valuable corporate asset.
Do Americans really pronounce bleach like that?
No. Everyone I’ve heard pronounces it with a long “e”, not short “e”.
Pretty sure he’s just goofing, because he does pronounce it correctly at one point.
No, as he mentions in a video description on his B channel, he’s mispronouncing it intentionally to annoy the people who don’t get the joke
Well he is half way to making mustard gas.
No, he isn’t.
YES HE IS!!!
making mustard gas is ridiculously easy. You can’t do anything cool with it. Unless you’re an industrial chemist you can’t use it for anything. Don’t try making it or you will end up hurting yourself and / or your loved ones. Even small doses can cause severe chronic respiratory problems. You’ll live with the consequences for the rest of your life however long or short that may be.
mustard gas manufacture would require some ethylene, and either a sulfur or phospho-chloride, and there is none here. perhaps his -next- video? of course, you could also quibble on the basis of “half way”; but i’d suggest that chloride gas and sodium gets you rather less than half way to 1-chloro-2-[(2-chloroethyl)sulfanyl]ethane (and yes, i’m a chemist, if not exactly industrial or industrious)
my bad :-( I was confusing mustard gas with phosgene. either way phosgene is very nasty
Interesting on a similar note that is bad stuff, I found mole killer poisons that had similar molecules to mustard gas and phosgene that are off the shelf at Home Depot. I wound up using traps and the stubborn ones… I yesterday ran the lawn mower, with not the smoothest lower idle, exhaust piped through a flexible electrical conduit into the mole holes for 15 minutes in each area where I suspect they are under some bush roots.
Before the internet, any knowledgeable person can walk into almost any well stocked university library and find books and how to do some of this stuff or at least the basic idea involved. Plenty of references for making sulfuric or hydrochloric acid.
True but when was the last time your local library was supported through advertising revenue and agreggating/selling your details?
Electrolysis of salt water to produce chlorine is done daily by tens of thousands of people.
This is the standard mechanism to sanitize a “Salt-Water pool”. In that case it’s optimized to leave the chlorine in suspension in the water and they use “a bit less” mercury. :-)
My understand is that’s the easiest way to produce sodium hypochlorite from electrolysing salt water long enough and with more than salt water and any excess chlorine can be drawn off for store and usage elsewhere in a slightly higher pressure containment than at atmosphere. I also understand it shouldn’t be done in pools for any length of time as it causes the water to become alkaline which degrades skin over time and causes eye irritation as the hypochlorite decomposes by out-gassing chlorine…
Not entirely sure that I understand your comment.
Salt water pools with a chlorine generator installed in the filter system is a quite common solution and absolutely intended for long-term use (years at least). Most of the people that I know that have them say that they are nicer to swim in and easier to care for than other methods.
The hardware is readily available in lots of places and there’s much written on the subject. Here’s a marketing-brochure version of how it works.
http://www.inyopools.com/Blog/what-is-a-salt-water-pool/
Ah well DKE welcome to chemistry; dilution, equilibria, molar calculations, rate of change, flowrate, contamination, out gassing, organic reactions, qualitative perception, analysis etc.
Pertinent link thanks and pretty much relevant though intended for general public, does have the relevant foundational factors ie. Amount of salt tiny, comparative size of device tiny, does mention the soft feel however = mildly alkaline.
Worth first becoming aware of the underlying equations in respect electrolysing salt water.
https://en.wikipedia.org/wiki/Salt_water_chlorination
The first part of my posting you replied to in respect of mechanism to produce chlorine gas – if you really wanted to ie. My point ‘more salt’ not directly applicable to a managed salt water pool and at that level is a chemical engineering issue in terms of intent to produce chlorine.
Last part of my post in relation to casual safety warning as extended use in pools without due care and attention and for some using too much salt without proper management controls and instrumentation can be irritating and harmful at the extreme end as it can trigger sporadic allergic reactions mostly skin and eyes. Btw. The soft feel due to higher pH (hydroxide) much like mild soapiness – very minor not considered any sort of long term problem, hardly noticeable for most part minus placebo effect. NB Some chlorine will evaporate too, hence the issue about adding small amount of hydrochloric acid offers two good aspects; reduces pH in case to address alkalinity and replenishes chlorine.
For what its worth one could approach an ideal by use of; some (iodised) salt around qty suggested in your link, controls to add appropriate amounts of hydrogen peroxide (to denature any DNA and free amines, urea etc) and ozone injection too. Fortunately last two can be done electronically with electronic control systems once initial salt added with only minor top-up. Also I’d add small amount of copper chloride along with the iodine in the iodised salt as both antibacterial :-)
NB ‘Difference between a poison and medication is so often the dose’ attributed to:-
https://en.m.wikipedia.org/wiki/Paracelsus
“a rail-gun for mercury” eh? ???? If I didn’t know better, I’d say Cody was…
…as mad as a hatter. ????
How to make the sodium chlorate would be interesting to see for a dancing gummy bear reaction.