[Jan] is one of those people who’s always playing around with synthesizers, and in this day and age, that means a lot of USB cables supplying power. If you want to make a synth setup portable, your best option is looking at USB powerbanks with their fancy lithium cells. These will work just fine, but remember: you can buy D cells just about anywhere, and they actually hold a ridiculous amount of energy. They’re cheap albeit one-use and disposable, so why not build a USB power bank out of a massive pair of batteries?
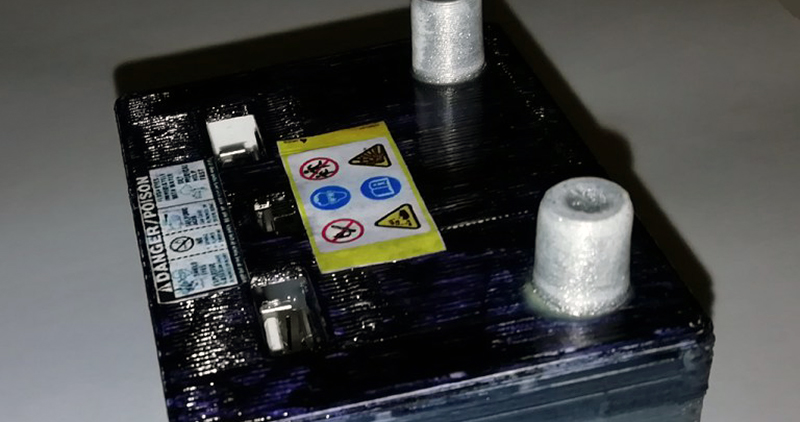
The build started off, naturally, with a pair of Energizer D cells that hold 20,000mAh. A battery holder for these cells is cheap and easy to source, leaving the only other needed component a cheap 5V boost converter. This was simply hot glued to the back of the battery holder in parallel, a simple switch was added, and the entire thing was fitted in a neat little 3D printed case that looks like a car (motorcycle?) battery.
Testing the with a phone revealed this thing will charge at 570mA from 3V, which is more than sufficient for [Jan]’s needs. Sure, using disposable batteries in 2018 is more than a little wasteful, but a project like this is meant to be a simple solution to the problem of providing power to USB devices anywhere. You can get D cell batteries everywhere, and what this build produces in damage to the environment is more than made up for in its convenience.














At best the environment to convenience exchange rate is undefined. In most cases it is very very high.
Does the exchange rate depend on what country you are in?
I assume it does not.
Kinda what I was thinking…. WTF does that even mean?
Yes, and on the personal preferences.
Like for me, i prefer using the car if it gives some time savings. I value my time more than the small environmental impact. I also prefer lightweight and non-fragile containers for my beverages (cans, PET bottles), but whenever possible I separate my garbage for recycling or at least dispose of it properly in waste bins, as I myself don’t like an unsightly littered environment.
It is understandable that using disposable batteries will produce a larger amount of waste product over time, but how much worse/better are the environmental impacts of producing and then eventually discarding a lithium ion pack? It is hard for me to imagine that the waste products involved in the production process and the waste material left in a discarded LiPo battery pack are more environmentally-friendly than those same aspects of alkaline batteries.
Sure, but you can recharge a lithium battery 300-500 times. So, it’s 1/500th of the waste.
Not to mention, Alkalines really suck in terms of energy / power / resistance.
Those batteries are rated at 400 milliohms fresh, so if you’re drawing 500mA at 5V (2.5W), which will be ~3W at the batteries, or 1A @ 3V. At 400 milliohms, that ends up being 400 milliohms * 2 * 1A = 0.8V drop (at fresh).
No wonder the phone is only charging at 570mA @ 3V (=~300mA @ 5V); the voltage rail is probably collapsing the second it tries to draw more.
Even at 570mA, you’re only getting a theoretical ~8000mAhr * 2 out of those cells, and that’s all the way down to 0.8V per cell. Based on his measurements of only getting 80mA @ 1.5V, you probably need more like 1V+ per cell (=2V total), which means you’re probably losing another 30-40% of the rated capacity. Now we’re at like 5500mAhr, which is easily into regular small lithium battery pack territory.
This article realty leave a lot to the imagination.
I might try something similar with a 7AH SLA battery.
Would be nice to have a “link” and see how this was done.
Here you are
http://blog.dspsynth.eu/diy-usb-emergency-power-unit/
Fixed. Thanks.
Thanks,
Normally [Benchoff] is quite malicious. I suppose he’s tired or stressed. Hope all goes well.
The printed car battery case is so cute. You should strip one end of the usb cable and put alligator clamps on the +5v and gnd wires, so you can clamp them to the “terminals” like you’re using jumper cables on a tiny car. Maybe a diode so nobody mixes up the polarity and toasts their device.
Also, isn’t it weird that we’re all sticking with mAh as the default battery capacity metric? It’s almost always in the thousands–why not drop some extraneous zeroes and use Ah? Is there some other reason I’m not thinking of, or is it just because the commas look more impressive when you’re advertising crummy counterfeit 18650s on ebay?
I think this has to do with innumerate consumers, who would choose a 10,000mAh battery over a 10Ah one every time.
Normies wouldn’t understand. If one sells for 20Ah vs 20000mAh then people will buy the one with more zeros.
My twenty billion femto-amp-hour battery should sell like mad then >:D
Not sure if you’re British or making a joke about how you could sell something worse by munging the units.
Like those 2000 Watt* speakers on ebay.
* for 2.7ns @ 250Amps when destructively tested
They’re the rubbish ones. Some last for 2700ps.
Damn !
You clearly won that round !
So 2000W is the maximum power you can reach when you burn (*) them?
*) with at least 90% oxygen.
2000W is actually the model number. ;)
I bought a 30 Amp solar regulator with the model number MPPT30A.
No, it wasn’t Maximum Power Point Tracking.
2000 megawatt speakers!*
*When struck by lightning while traveling at relativistic speed through a nuclear reactor. 5 watt RMS.
Do “Normies” even see those numbers anyway? My guess is they MIGHT notice the marketing claims of “x hours of talk time”, if they are particularly savvy shoppers. That’s probably about it. mAh, Ah, “jigawatts”, whatever…I bet their brains filter it right out as “techno babble” shortly before it reaches any decision making portion of their brains and waaaaaaaaaay before it reaches any conscious part.
Ah raspberries, the ol 18650 grift…
I agree tho the mini car battery with clamps would be adorbs :)
Kinda not very content-ful without a link…
That said, anybody who claims they can extract 20 Ah out of a D cell is either a charlatan, delusional, or has deep enough pockets to buy lithium primary D cells.
Energizer and Panasonic actually publish their battery datasheets. They’re kind of hit-and-miss, but they give enough data to show that at the discharge rate mentioned above (570 mA = 2.85W) into the phone, where you’re very likely sucking over an amp out of a pair of D cells, they will last less than 2 hours (“heavy duty”) or 5 hours (Alkaline). TWO (or 5) amp-hours, not twenty.
Still works out to about a penny per minute (if you buy your D cells in bulk.), about 5x more than I pay for electricity to run my whole house.
(And just because I am a pedantic ass: “20,000 mAh” is not a measure of energy. What’s wrong with saying it can deliver “10 watt-hours” (over 10 hours)? It’s correct and far more useful.)
If you read the article you know this is not for charging phones even if I tested it with a phone.
It is for powering USB gadgets and they dont pull >500mA from 3V.
50-100mA in my case and at that rate it lasts more than 2 hours.
And what to do with a fancy Li-Ion or Li-Po powerbank when its empty and nowhere to charge?
“And what to do with a fancy Li-Ion or Li-Po powerbank when its empty and nowhere to charge?”
Really? You are asking that? I pack it away to come home with me and charge it again when I get there. What do you do? Toss it in a creek?
Not less than with this power bank when it’s empty and you don not have spare batteries or an open shop nearby. But I still can charge the rechargeable one in the car, in a train, with a solar panel or at the airport (OK, there are also shops). For me it’s normally easier to get power than batteries.
With watt-hours you have to factor in the voltage, it’s another conversion to do, simpler to just stick to Ah or mAh or whatever. And yep it’s usual to state “at 1C” or the like for battery capacity, though again for a practical figure the discharge rate should be the one that’s actually occurring in use, it makes a big difference.
No, it’s the other way round: What counts is the energy and with Ah you have to factor in the voltage.
Worst case (or outright lie): Somebody sells a “7000mAh” 12V LiPo emergency starter battery for the car – consisting out of 3 2300mAh cells connected in series.
Three series 2.3Ah cells are 12V @ 2.3 Ah
Three parallel 2.3Ah cells are ~4V @ 6.9Ah
You can’t have your cake and eat it to.
That’s kinda the point. In the land of marketing wank they don’t have to say either of those things. They just plaster a “7000 mAh!!!” on the box (usually in an oval shaped balloon at a jaunty angle).
Check those numbers. 570 mA at >3 Volts< , not 5. 1.71 Watts. Going into a boost converter at typical 85% efficiency will yield about 1.45 Watts available at the output, or roughly 290 milliAmps.
Brian’s wording in the summary here says “Testing the[sic] with a phone revealed this thing will charge at 570mA…”, which I think it’s fair to assume means it charges a phone at 570mA (when running from a 3V supply). The original source wasn’t available at the time, so there was no way to do anything but take it on faith the Brian stated that correctly.
But you’re right. Now that we can see Jan’s the original article, we now know he said it draws 570 mA from the batteries while charging the phone. Interpreting the Energizer datasheet, it looks like you’ll get that 570 mA at around 1.3V, so the batteries are delivering 1.5W, and the converter (at 85% efficiency) is delivering 1.26W = 0.25A. That will take about 14 hours to charge the phone pictured. Which is about the maximum time the D cells will last.
So, two “20 Ah” D cells are enough to charge one 3.4 Ah (ok, “3400 mAh”) phone battery. Excellent.
(Since the CE8301 converter he used poops out at around 180 mA output, Jan must be stating the sum of the two converters he used in parallel.)
At least 10 years ago, Energizer sold essentially the same thing: an emergency cell phone charger that used a pair of 1.5V Lithium AA cells, boosted to 5V. Essentially indefinite (20 year) shelf life: throw it in your glove compartment for when you needed it.
They were in the bargain bin here at a supermarket 5 years ago for less than 5 bucks, but I see Amazon still sells ’em as new (not going to provide a link, but they are ASIN B000JD09P4 “Energizer Energi-To-Go” ).
A 99p shop a few years ago had them that take a single AA. A tiny PCB the same radius as the battery sits at the top of a nice metal canister, with a teeny boost circuit and an indicator LED on it. From there there is a USB-micro plug on a wire.
It was invaluable when my old phone’s battery would only hold a couple of hours’ charge, while I ordered a new one. Also good for camping, etc. It’s only slightly larger than the AA itself, so you can keep it in the same pocket as your phone. I bought 2, I wish I’d got more.
This lithium primary cells, which are “artificially” suppressed to 1,5V (1,8V) are ridiculously expensive. Rechargeable LiIon 18650 do not cost more and have also extremely low self discharge. I would prefer them. And regarding “throwing into the glove box” I see a cigarette-lighter to USB adapter more useful.
I would like to know about the criteria for moderation. I can not explain, what triggered this.
This one isn’t much better than the belt-belt article recently…
Hey, wait! I thought that was the best article all month.
How can you even say “belt-belt” without smiling?!?
Some people have no sense of humor.
;)
“what this build produces in damage to the environment is more than made up for in its convenience”.
Well now, there we have the road to hell spelled out in plain english.
Better than the “road back to stone age” the environmentalists wish us to go.
The last time I checked, a 2-pack of name-brand alkaline D-cells cost $US 4.95 (and up), depending on where they were bought, and the brand name .
As a consideration to the environment, my wallet, and my serious concerns about trusting long-term power drain (while maintaining output voltage) to “D” cells, my design for this application would be based on a $10.90 (at Amazon), 4.5 Ah sealed lead-acid rechargeable (SLA) battery. At these kinds of current drains, a garden variety, high-current (5-10 A) silicon diode will provide about a 1-volt drop, to provide ≈ 5 vdc at the output. If you have a serious case of OCD, you can buy a buck converter for $10 – $15 (…it even comes with an LED or LCD display; one even comes with a USB output!) to give you exactly 5 volts. (And don’t forget–a “boost converter” is a fixed cost of the design which is being offered.)
…And if a 4.5 amp-hour battery is not large enough, you can always get a 6 v., 12 Ah SLA battery for $18.00.
It won’t take “burning through” many of those throw-away D-cell pairs to pay for this environmentally-friendly and much more sound solution.
[Simply an aside– two “D” cells are good for 20,000 maH, or 20 amp-hours?. Perhaps…for 30 seconds–you’re not being told the entire story here..]
It’s been my experience in working with energy sources over the years that there is somewhat a direct relation between the amount of energy one can store in a device and its volume. Simply compare the volume of two “D” cells with the volume of the suggested SLA battery / batteries.
Just sayin’…
I’ve read that an SLA battery over it’s entire lifetime will only store 2x the energy it took to make. That, combined with the lead issue, makes me think that properly recycled D batteries might not be much worse.
Lead acid can be destroyed just by letting it self discharge too much. They’re fussy, heavy, pieces of junk. The best thing about them is that you can float charge them, which is why someone should make a smart controller that lets you do the same with LiFePo4.
IMHO LiFePo4 batteries are the way to go for the stuff you’d normally use lead acid for, but 4.5Ah is small enough that the newer lithium titanate is an option. Those give 5000-20k cycles.
“…properly recycled D batteries might not be much worse…”
From what I have observed, as far as most people are concerned there is nothing more disposable than a dry-cell battery.
“,,,combined with the lead issue…”
Most lead-acid batteries–compared to dry-cells–are disposed of properly, and the lead is recycled.
“…Lead acid can be destroyed just by letting it self discharge too much…”
NO battery should be allowed to self-discharge. If you don’t provide a maintenance charge regimen for any rechargeable battery, you don’t understand batteries, and will pay for this lack of knowledge by continually replacing them.
“…The best thing about them is that you can float charge them…”
Absolutely true, using a proper float charger. They will last indefinitely this way.
“…LiFePo4 batteries are the way to go for the stuff you’d normally use lead acid for…”
Let me know when one’s available for my car for $125, with an 800 to 1000 CCA (cold-cranking amps) spec with the reliability of lead-acid, and then I’ll listen.
Now consider the fact that your automobile battery lasts for years; your
computerelectronics backup power-supply equipment lasts for years, and all those “EXIT” signs and emergency power-outage lighting systems in all public buildings work for years. All powered by lead-acid batteries. There simply is nothing better, all the promises of new battery technology notwithstanding.One of the founders of Intel is famous for a statement which goes something like this–
“The best, most reliable, cheapest part for the job is the one that doesn’t quite exist yet…”
Dealing in speculation is good. Sometimes reality–which oftentimes consists of years and years of experience–rears its ugly. pesky head.
That’s the one thing lead-acid has over other batteries, AFAIK. That lead is valuable, and easily extractable in quantity from old batteries. Scrappers will pay for old lead batteries. So recycling has been part of their life cycle since they first hit the public.
They’re good for what they’re good for. They’re robust and last a long time properly cared for. OTOH they’re hardly lightweight or portable. Mobile phones didn’t take long to ditch them, as soon as Nicad was an option. For car batteries, they’ll probably last as long as the internal-combustion engine does.
And lead acid doesn’t explode. That’s always a good thing!
The exit signs are also often powered by NiMH type batteries. “Nothing better” is, wrong, I would say “nothing cheaper”. A LiFePO4 IS better, it is just much more expensive, my 12V 100Ah LiFePO4 cost about €750,- , you can convert to US$ yourself. It is even spec’d for 1000A for 5 to 10s. The good thing is: You get 1000cycles 100% depth of discharge (or 3000 with 80%). Compare this to lead acid, where you should not go below 50% discharge, if you want to get a decent amount of cycles, I think, if you want more than 100 or 200.
There are LiFePO4 replacement batteries for car batteries, but most time they have much less capacity than the lead acid counterpart they replace, probably just because they are much more discharge tolerant. What I am not sure is, how they react to automotive temperatures. My LiFePO4 battery wants to be charged only between 0°C and 45°C
Can’t really argue with the cost issue. There’s probably some hybrid lithium ultracapactitor based system out there that can be made affordable, but Lead Acid is cheap as dirt in some applications.
For anything that will be exposed to cycling, LiFePo4 is actually cheaper per total watt hour over the life of the battery IIRC. And below a certain size battery cost becomes irrelevant compared to the cost of the rest of the system, and a $20 RC hobby charger works fine if they aren’t on standby.
LiFePo4 is lighter, stores more energy, self discharges much slower, and tolerates deeper discharge. IIRC it can even be float charged (with *very* accurate voltage control control).
I haven’t actually owned any LiFePo4 batteries that long, but I have heard 10-15 years is normal for them in some applications, and I’m pretty sure the even newer titanate version goes even longer.
They might be completely impractical for the average consumer or installer of emergency lighting because of the price and more complex circuitry, but I’d suspect the average ham radio guy or DIYer working on anything portable I willing to pay a little extra for something that’s lighter and going to last longer.
I once had a “ghetto blaster” type cassette radio recorder, which needed 8 D-cells. For the same price as this 8 D cells I got a 7Ah SLA battery. I t is true, that this primary cells have about double the Ah capacity, but after 2 charge cycles they were equal. And assuming that energy use during manufacturing is somehow related to manufacturing cost and thus retail price, I do not really believe that bad energy ratio you cite. The lead was no issue, it stayed happily in the battery until it’s EOL and recycling. Not so the acid, Some drops somehow found a way out and damaged a pair of jeans and a chair.
LiFePo is really very nice and has 1000s of recharge cycles and it’s voltage also fits nicely to 6V or 12V lead acid applications ( 7,2V or 14,4V end of charge voltage). The mean voltage is about 10% higher, above 13V for most of the time. This increases power efficiency with ordinary PWM solar charge controllers.
No. A high current Si diode will not have 1V drop at 1/10 od it’s rated current. Please invest another $ into a low dropout linear regulator.
Apart from that I also strongly prefer rechargeable (best lithium) power sources.
I had to read this three times and then still couldn’t believe what I read.
“and what this build produces in damage to the environment is more than made up for in its convenience”.
Why? This is the approach of most normal people to environmental protection issues, it’s just honest. Only hardcore environmentalists who give the issue of environmental protection the highest priority do not value their convenience.
We can not influence the climate anyway. So it is better to think about how we adapt to it’s (natural) changes, than to get back to pre-industrial stone age in the hope we have any influence.
Alkaline cells aren’t especially damaging to the environment, any more than other products of that mass. So we buy stuff and throw it away. That’s why we have garbage cans and landfills. Life goes on.
Here is a link:
https://www.hackster.io/janost/the-ultimate-mega-power-bank-7e8d19
“A battery’s capacity depends upon its cell chemistry and current draw. Energizer brand rates its alkaline D cell at approximately 20,000mAh at 25mA draw, but estimates performance closer to about 10,000mAh at 500mA draw.”
source: duracell
This one begs for critical analysis, and we all should be glad you took the time required to submit it…
I have no doubt that The Manufacturer [no names] claims an estimated performance of
10,000 mAh when supplying 500 mA. Let’s re-cast that as 10 amp-hours at 1/2 amp draw. Under the ‘rules of the game’, this does mean that their D cell will provide 1/2 amp for twenty hours; kudos to the manufacturer for his honesty in de-rating the capacity as the current demand increases.
I’m not going to invest in the high cost of D cells–any more– to check this out, but only rely on experience. All the fluorescent emergency lights I’ve ever owned (and I’ve still got a few)–which used D cells–would not run–last–for 20 hours on a set of D cells, but more like 3 hours at best. (I also know, from experimentation–that the current drain of this type lamp is ≈ 500 mA). (I live in an area where power outages have given me much too ample opportunity for un-wanted data-gathering, and D-cell purchases).
Take it with a grain of salt when you see an advertisement for an automobile “Jump-Starter’ which fits in the palm of your hand. My two ‘jump-starters’ contain two ‘gel-cel’ s of 18 Ah and 24 Ah capacity…which have lasted for years, by the way.
Thomas Edison said something akin to, “Creation of a new battery technology brings out man’s latent capacity for lying.”
Caveat emptor.
Rechargeable, means you have to remember to charge them, also don’t last long, if you don’t maintain the charge, if you don’t use them much. A musical instrument, I’m sure ideally you want to plug it in to the wall, but not always available. The convenience is that you can buy charged replacements, at a convenience store in a few minutes, most anywhere, an acceptable delay. Rechargeables, you could carry spares to swap out, but then again, if you are prone to forgetting to keep them charged, or maintain them, might not last long enough. You wouldn’t want to be performing for money, and lose power in the middle of a song. A glitchy performance, is amatuer, and not good for promoting yourself for future work.
Look into the self discharge rate of lithium ion cells. There’s no reason you can’t leave them for long periods, if your circuit isn’t drawing power from them while idle. I have a geiger detector which is powered by a single 18650 cell. I’ve left it mostly idle for about 2 years, but I can still pick it up and know that it will turn on and work, because the cell still has plenty of charge.
The two-way radio Yaesu FT-817 has a ni-mh rechearcgeable battey pack, but you could swap it and put in 8 AA cells alkaline batteries (or use a 12V external supply).
I was thinking why not use a 6V lantern battery (there are rated 11 Ah http://data.energizer.com/pdfs/1209.pdf)? You still need a stabilizer if the devices doesn’t like 6V. Old TTL/CMOS board worked with na 4,5V battery, and also the Arduino works fine (using the direct 5V connection). Other systems I don’t know.