When was the first nuclear reactor created? You probably think it was Enrico Fermi’s CP-1 pile built under the bleachers at the University of Chicago in 1942. However, you’d be off by — oh — about 2 billion years.
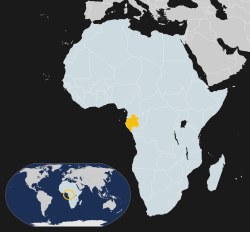 The first reactors formed naturally about 2 billion years ago in what is now Gabon in West Africa. This required several things coming together: natural uranium deposits, just the right geology in the area, and a certain time in the life of the uranium. This happened 17 different times, and the average output of these natural reactors is estimated at about 100 kilowatts — a far cry from a modern human-created reactor that can reach hundreds or thousands of megawatts.
The first reactors formed naturally about 2 billion years ago in what is now Gabon in West Africa. This required several things coming together: natural uranium deposits, just the right geology in the area, and a certain time in the life of the uranium. This happened 17 different times, and the average output of these natural reactors is estimated at about 100 kilowatts — a far cry from a modern human-created reactor that can reach hundreds or thousands of megawatts.
The reactors operated for about a million years before they spent their fuel. Nuclear waste? Yep, but it is safely contained underground and has been for 2 billion years.
The Basics of Fission Reactors
Any fission reactor basically works the same. An unstable atom like uranium or plutonium breaks apart, creating fast neutrons. There’s a chance some of those neutrons will strike nearby unstable atoms, causing them to break apart too. However, because these neutrons are fast, statistically this won’t happen very often. However, if a moderating substance like water or graphite slows down the neutrons, they have more time to interact with other unstable atoms, so more atoms break down.
If you balance it right, the atoms breaking apart will cause a chain reaction that sustains itself. That is, neutrons hit atoms, releasing more neutrons, which hit more atoms. The heat released is substantial and we usually use it to make steam to drive an electric generator.
Nuclear Fuel Used to Be Much Better
 The original CP-1 reactor used natural uranium and so it took a lot of fuel since ordinary uranium is relatively stable. If that uranium is refined into higher concentrations of specific isotopes, a more powerful reaction is possible with less fuel.
The original CP-1 reactor used natural uranium and so it took a lot of fuel since ordinary uranium is relatively stable. If that uranium is refined into higher concentrations of specific isotopes, a more powerful reaction is possible with less fuel.
There are three isotopes of uranium: U-238, U-235, and U-234. Uranium decays naturally, and highly active isotopes are now in short supply. Apparently, whatever process created the uranium on Earth, it all appeared at the same time (and has been decaying ever since) because no matter where you mine uranium today it is about 99.275% U-238.
U-235 is better for reactors but, today accounts for only 0.72% of what comes out of the ground. We enrich uranium artificially to have about 3% U-235 for use as fuel. Scientists think that when the Earth formed, U-235 was 30% of the crust’s uranium. That number keeps falling, of course, and 2 billion years ago, the proportion would be about 3.6% — just right for nuclear fuel.
When Mined Uranium Came up “Light”
In 1972 a French team mining uranium in Gabon noticed something strange. The ore didn’t have the expected 0.72% of U-235 but instead had 0.717%. That sounds like a tiny bit — and it is — but U-235 content is remarkably consistent all over the world (and on the moon, too). Further investigation confirmed that the missing U-235 was fissioned away in a natural nuclear reactor. In particular, all five fission products you’d expect were located. The amount of Xenon gas trapped in the reactor made it possible to calculate the operating cycle of the reactors.
Eventually, they would find 17 of these natural reactors. You might wonder why there are not even older natural reactors. After all, if 3.6% is a good fuel, wouldn’t 5% or 10% be even better?
The conditions had to be just right. A little over 2 billion years ago, there wasn’t much oxygen in the Earth’s atmosphere and this made it difficult for uranium to concentrate. So to get a natural reactor you need a concentration of uranium that has a high percentage of U-235. There also needs to be a suitable geometry of the material, a moderator like water, and no neutron-absorbing material in the area.
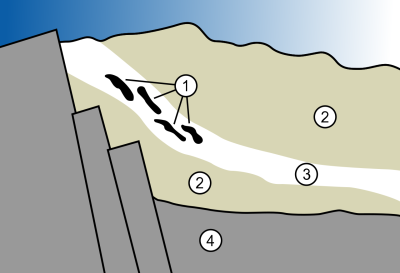
You can see in the figure that the uranium ore (3) is surrounded by porous sandstone (2) all on a layer of granite (4). The black zones (1) represent some of the natural fission zones.
Enough water would seep down through the sandstone and cause a reaction that would boil off the water within a half-hour. The reaction would then stop until sufficient water seeped in again creating a 3 hour total cycle time. This allowed the reactors to be very stable and appears to have allowed the reactors to run for about one million years.
There could be more reactors yet to be found or that operated and then did not survive the intervening 2 billion years for us to find. Conditions in Gabon were also just right to preserve the reactors.
Modern Times
If you are worried this could happen today, don’t fret. The U-235 content today is insufficient for this to happen again naturally. However, scientists have studied how waste products have been contained as it might lead to better ways for us to store nuclear waste we are creating.
As an interesting side note, there is a controversial theory that the moon was blown out of the Earth about 4.5 billion years ago by another natural reactor near the edge of the Earth’s mantle. While this hasn’t been disproven, as far as we can tell, most scientists seem to accept that the moon formed due to an impact on the early Earth.
















I just watched a video on this same subject: https://www.youtube.com/watch?v=pMjXAAxgR-M
This professor has a bunch of interesting content if you like to see how our various energy systems works.
Thanks for the video, very interesting.
:o)
At the end of the video there is a link to a photo of such a natural nuclear reactor:
Oklo: Ancient African Nuclear Reactors
https://apod.nasa.gov/apod/ap100912.html
A lot of the earth’s heat is from friction , and there is leftover heat from when the planet was formed. I don’t know the percentage of each. Here’s a good article on the subject: https://www.scientificamerican.com/article/why-is-the-earths-core-so/
What about the core of the earth, there is one massive massive fission reactor there. But lucky for us there is so much shielding under our feet, we do not need to worry about it. And that reactor is powering the earth’s magnetic dynamo, which lets earth have a thick breathable atmosphere. Of course the heaviest elements are trapped unable to escape the core to the surface due to the intense gravity holding them in place.
The core of the earth is hot iron and nickel. Some heat is generated by decay, but there is no fission reactor down there…
Yes and no. The earth’s core is heated by spontaneous fission, as is the whole rest of the earth anyhow – this is not a nuclear reactor any more than a lump of uranium itself is a “reactor”. It just keeps decaying with its natural half-life and produces heat as a byproduct.
That’s almost entirely alpha and beta decay. Spontaneous fission accounts for about 0.005% of uranium decay, and essentially nothing for thorium or the rest of the decay chain.
Sorry, what I meant is just regular radioactive decay. I didn’t remember spontaneous fission was its own thing.
So are you saying that J. Marvin Herndon is wrong ? There there is not a (~3 TW) Georeactor at the core of the planet. Or are you saying that a Georeactor is not a fission reactor, even though it generates heat by fission. I’m confused.
If he’s right he’s wrong. In that all fissionable material in the core would have got used up billions of years ago.
That can’t be true, U238 has a half-life of 4.468 billion years :) But yea anything that burns hot, would have burned up long ago, but under the extreme pressure is it possible that the cooler surrounding lower radioactive elements are continually recharging the smaller hotter higher atomic number elements at the core.
“That can’t be true, U238 has a half-life of 4.468 billion years :)”
Reactors burn through their fuel faster than the decay timescale – if they didn’t, they wouldn’t be reactors, since there’d be no reaction. However Herndon’s hypothesis was a bit touchy in exactly how long the reactor could actually run – one of his papers was basically suggesting that it’d be running out essentially any day now (however, with the much lower power imposed by antineutrino studies, it could obviously last for far longer).
Usually “fission reactor” is defined as having controlled nuclear chain-reaction. Nuclear bombs are not reactors because the chain reaction is not controlled. Georeactor and RTGs are not reactors because there is no sustained chain reaction, just spontaneous fission.
Herndon’s “georeactor” is the idea that there’s enough uranium at the Earth’s core to produce a literal reactor. This idea is partly intended to explain He-3 venting from the Earth’s interior. So yeah, it actually wold be a sustained, regulated reaction.
However, the specific reactor initially suggested by Herndon would have power levels above 3 TW, which is excluded by antineutrino data. Of course, like any good theorist, Herndon just treated that as implying that the power level is 3 TW, right at the limit, but the antineutrino constraints continue to push that down – at this point the power must be less than 2.4 TW at the 95% confidence level. As that level decreases, the original reason for proposing the hypothesis (to explain the elevated He-3/He-4 ratio in vents) goes away.
“So are you saying that J. Marvin Herndon is wrong ? There there is not a (~3 TW) Georeactor at the core of the planet.”
Yes, he is wrong (at least, his specific hypothesis in the paper). That hypothesis was rejected (at >95% CL) by KamLAND and Borexino about a decade ago.
It also should be noted that the total heat flux of the Earth is like, 47 TW, and that’s very well measured. So even if there is a sub-3 TW reactor at the Earth’s core, that’s massively subdominant to almost all major sources of heat on the planet (hell, tidal heating from the Moon likely causes more heat than that).
And in fact, even the core isn’t a significant source of radioactive decay heat. That comes from the mantle and crust. Most of the heat that comes from the *core* is actually *primordial* heat, left over from the initial formation of the Earth (and some early nuclear decay too, obviously), and that’s somewhere between 5-15 TW, still well more than 3 TW.
It’s estimated that about 22 TW of that is caused by simple radioactive decay all over, and the rest is leftover heat.
The core of the earth is not considered a fission “reactor” as such.
Most of the core heat generated by far comes from singular emissions usually (though not necessarily always) beta decay of Potassium-40 and thorium and recently discovered some bismuth as there is no stable isotope, there could well be some fission of U-235 if any left but, it’s not classed as any sort of reactor in conventional terms, it’s negligible spontaneous fission depending on the branches of the decay chain which is of course dependent on permutations within the mix of materials at the core eg one decay product affecting others etc for which I also consider is negligible given the comparative energies involved…
Of course the oldest fusion reactor in closest proximity is of course the Sun, which interestingly exploits the C,N,O catalysis of Hydrogen to Helium for energy production via tunneling hence it can produce energy without needing high temperatures as here on earth as sun as high core pressure. Fwiw. We produce more (chemical) heat per unit volume in our bodies than the suns core (nuclear) heat output for that same volume…
“Most of the core heat generated by far comes from singular emissions usually (though not necessarily always) beta decay of Potassium-40 and thorium”
That heat comes from the *mantle*. The heat flow from K40 in the Earth’s core is tiny (less than 0.2 TW) because potassium basically gets pushed out. Same deal with thorium.
The Earth’s core is basically just cooling due to heat from its initial formation.
“which interestingly exploits the C,N,O catalysis of Hydrogen to Helium for energy production via tunneling”
Also, the CNO cycle isn’t that important for the Sun – by the standard solar model, it’s only a percent or two. The vast majority of the hydrogen burning in the Sun is via the pp-chain (which does occur via tunneling, mind you, the temperatures in the Sun are not high enough to classically bridge the Coulomb barrier). Measurements of exactly how much CNO cycle production is occurring in the Sun are an active field of research: the pp-chain neutrinos have been measured (all except for the extremely rare hep branch), but the CNO cycle neutrinos (though predicted) have not been seen yet (predictions are about a factor of 2 below current limits).
You need higher temperatures for the CNO cycle to take off (about 16-17 MK, so ~30% higher mass than the Sun).
Wow, that was interesting to read, thank you!
there was an article the the August 1967 issue of Scientific American I believe
July 1976. I remember reading at the time, and have been keeping clippings ever since. A friend worked for BBC Science at the time and they did a piece for Radio 3. I must ask him if he has a copy of the recording.
Here is a followup from SciAm in 2009: https://www.scientificamerican.com/article/ancient-nuclear-reactor/
The photo of the old “reactor” reminded me of this old Outer Limits episode that scared the bejeezus out of me as a kid growing up watching it afterschool.
https://www.youtube.com/watch?v=mYqCKYpBl7s
I think the sun counts as a reactor, and it’s a bit older than 2 billion years.
The Sun is a Fusion reactor, this was a Fission reactor. Not the same or even similar.
Except for turning matter into thermal energy, completely different.
I think that’s always been a driving force on this planet. Both from the planet itself, and from space.
It has been more than 30 years that ANDRA (French Agency for Nuclear Waste Management) has communicated on OKLO in the context of deep disposal safety over time.
I’ve noticed that the number of results from Google for searches has been increasing over the last 20 years. Partly because of studies of sites for waste storage, but also for studies of whether there have changes in any of the nuclear constants over the intervening period.
The Yucca Mountain (Nuclear Waste Repository) Project studied the Oklo area reactors in great detail in order to help validate codes for nuclear isotope migration from the proposed repository. The details can be found in the License Application which should be available via the NRC Adams database. David Stahl, Ph.D.
Wikipedia lists 8 isotopes of uranium; I wouldn’t be surprised if there were more. 3 are considered natural.
You guys are a great read! I just happened to stumble upon this by mistake and started reading.well hello sir I spent all Afternoon reading this and from sites before keep it coming.
For some reason since the nineties a lot of people started going in to nuclear engineering. I studied it long enough to know some things, but evidently there was some boom in the industry..
Which is strange because the required materials are highly illegal in just about every nations without extensive and expensive permits and licenses.. You basically have to go in to government contracting to even work with fission or do fusion R&D…