Sulfur hexafluoride (SF6) is not nearly as infamous as CO2, with the latter getting most of the blame for anthropogenic climate change. Yet while measures are being implemented to curb the release of CO2, for SF6 the same does not appear to be the case, despite the potentially much greater impact that SF6 has. This is because when released into the atmosphere, CO2 only has a global warming potential (GWP) of 1, whereas that of methane is about 28 over 100 years, and SF6 has a GWP of well over 22,000 over that same time period.
Also of note here is that while methane will last only about 12.4 years in the atmosphere, SF6 is so stable that it lasts thousands of years, currently estimated at roughly 3,200 years. When we touched upon sulfur hexafluoride back in 2019 in the context of greenhouse gases, it was noted that most SF6 is used for — and leaks from — high-voltage switchgear (mechanical switches), transformers and related, where the gas’ inert and stable nature makes it ideal for preventing and quenching electrical arcing.
With the rapid growth of highly distributed energy production in the form of mostly (offshore) wind turbines and PV solar parks, this also means that each of these is equipped with its own (gas-filled) switchgear. With SF6 still highly prevalent in this market, this seems like an excellent opportunity to look into how far SF6 usage has dropped, and whether we may be able to manage to avert a potential disaster.
Best at Not Doing Anything
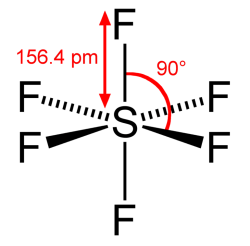
What makes SF6 such an excellent, one-stop shop choice for quelling electrical arcs and insulating high-voltage electrical system is because of its stability. Generally, it does not readily interact with other substances, which leads to its properties of being colorless, non-flammable and non-toxic. Unfortunately, this lack of chemical reactivity also means that it can hang around in e.g. the Earth’s atmosphere for a very long time.
Although SF6 occurs naturally, the overwhelming majority is produced by humans, for use in industrial processes and medicine, but primarily in high-voltage electrical systems as a dielectric gas. The main purpose of a dielectric gas here is to increase the breakdown voltage so that higher voltages can be used in less space, generally relative to air.
For when some arcing does occur, the purpose of the gas should also be to quench the arcing, which is where SF6 shines. Although a small part of the gas may be broken down into the toxic S2F10 (disulfur decafluoride), most breakdown products will quickly reform into SF6, which makes it a low-maintenance choice for switchgear. Especially for gear that ends up being installed somewhere remote and relatively inaccessible, this is a very helpful property.
Because SF6 is non-toxic and has a high molecular weight, it has also found use as an inverse party gag to helium: where helium’s low molecular density makes for an increase in perceived pitch when speaking through a helium-filled medium, breathing in SF6 will significantly lower the pitch of one’s voice until the gas has been expelled from the person’s airways.
Gases Want to Be Free
An unfortunate side-effect of our planet’s gaseous atmosphere is that any gases which escape from containment, or which are released through human activity end up joining said atmosphere. How concerned we should be about this depends on the gas in question. When CFCs were found to be rapidly eroding the Earth’s ozone layer, this made it crucial to immediately eliminate any significant release of this gas. This was accomplished via the Montreal Protocol, which saw a rapid cessation of most uses of CFCs.
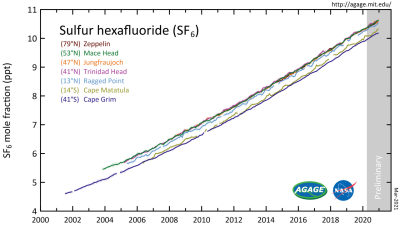
In the case of SF6, it would seem fair to ask just what the scope of the threat is. To assess this we can look at AGAGE’s data. This is the Advanced Global Atmospheric Gases Experiment, which keeps track of a wide range of gases in the atmosphere. Their findings are that the amount of SF6 has significantly increased since 2000, increasing from about 4 ppt (parts per trillion) to around 10 ppt by 2020, with a linear increase becoming noticeable around 1970. Pre-industrial troposphere levels were roughly around 54 ppq (parts per quadrillion).
As over 80% of the SF6 that is produced is used in the electrical power industry, this is also not surprisingly the biggest source of leaks. Much of this is due to the distributed nature, instead of the gas being used in a closely monitored industrial process, items like switchgear are located literally around the world, in deserts, at the top of wind turbines and in the middle of fields. When being installed, repaired or decommissioned, switchgear can also be damaged, with SF6 gas escaping into the atmosphere.
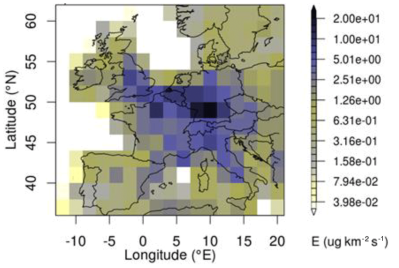
In a 2020 study based on the AGAGE findings titled The increasing atmospheric burden of the greenhouse gas sulfur hexafluoride (SF6), Simmonds et al. cover the past 40 years of measurements. They note five main source of SF6 leakage:
- Electrical power industry
- Magnesium industry
- Aluminium industry
- Electronics industry
- SF6 production itself
As for the major SF6-emitting countries, these were deduced from measurements to be primarily China and South Korea in East-Asia, and Germany in Western Europe. In the case of Germany semiconductor producers are suspected of being major contributors.
As for high-voltage gas-insulated switchgear (GIS), these use as mentioned >80% of the annual production of SF6, with medium-voltage GIS another 10%. These GIS tend to have a lifespan of 30-40 years, with new SF6-based GIS being installed even today, each of which will suffer some level of leakage during normal operation due to the imperfect nature of seals. In the magnesium, aluminium, and semiconductor industries, leaks have been gradually reduced over time, but are still a significant source.
In 2018, global emissions of SF6 were 9.0±0.4 Gg yr−1, with 2018 CO2 emissions being 33.1 Gt (33,100,000 Gg). Taking into account the much higher GWP (22800) of SF6, this makes its 2018 emissions equivalent to about 205,200 Gg, or 0.6% of annual CO2 emissions. While not an astounding number, we must take into account here that so far the emissions of SF6 are increasing year over year. Any SF6-based GIS or similar installed today will be adding to this total for the next decades, while contributing to global warming for a longer period than the industrial era so far.
Alternatives
Clearly, replacing SF6 and generally preventing it from leaking into the atmosphere is a good thing, then. Perhaps ironically, SF6 previously replaced the use of oil in switchgear due to toxic and otherwise harmful substances, and some of the suggested replacements for SF6 are themselves not as benign as this gas. Where possible, one of the best options is a vacuum, with a high vacuum providing very high dielectric insulation.
Maintaining a high vacuum is not easy, especially not over years, leading to alternatives ranging from plain air, CO2, and various fluoride-based substances. Recently Owens et al. (2021) as researchers at 3M published a study on two SF6 alternatives which 3M sells commercially. Their commercial names are Novec 4710 ((CF3)2CFCN) and Novec 5110 ((CF3)2CFC(O)CF3), both being fluoronitrile and fluoroketone mixes.
The idea is that such mixes are added to CO2 or air inside the GIS, to improve the dielectric properties. In this configuration, Novec 5110 with air mixture looks pretty decent, with a (100-year) GWP of <1, but Novec 4710 with CO2 mixture has a GWP of 398, which is better, but not great. SF6 also showed an overall better cold weather performance, down to -38 °C, compared to -27 °C for Novec 4710/CO2, and 0 °C for Novec 5110/air.
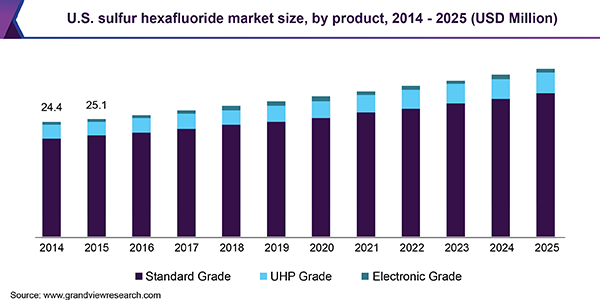
This highlights the complexity in replacing SF6 in GIS applications, as each part of an electrical grid has different temperature ranges and other factors that would be a particular SF6 alternative more attractive. With SF6 being relatively cheap, universally applicable, and its use so far unencumbered within the electrical power industry — even within the EU’s F-gases regulations — it’s little wonder that the SF6 market keeps growing year over year.
Not Just SF6
The fluorinated gases have in common that they tend to be man-made, popular in industry and other applications, and have a high GWP. They include HFCs, PFCs, SF6 and NF3. Of these, HFCs are popular in refrigeration, where they replace the previously popular CFCs, along with a number of other gases. Through their production, use and eventual decommissioning, a significant amount of these gases end up in the atmosphere, where they contribute to the specter of anthropogenic global warming.
Looking at the popularity of these gases, the difficulty in finding replacements, and the push to produce more and ever cheaper refrigerators, wind turbines, and distributed power systems, it seems unlikely that we’ll be seeing a major change here. Meanwhile every day sees more of SF6-based GIS and kin being installed in the world’s rush to decarbonize and expand the electrical grid, where they’ll continue to be a problem for decades to come.
Although this is a perhaps depressing perspective, some hope can be gained from the way the world came together to banish CFCs when it was clear that they formed an existential threat to all life on this Earth. Here is to hoping that we can do that a few more times.
















So, I guessed I missed the part about _how_ SF6 contributes to AGW.
IR cross-section of SF6 is 2 orders of magnitude higher than CO2. More bonds in the atom -> more IR absorption -> more heat trapped -> more warming / molecule.
Cool!
Oh, I mean, interesting!
Thanks
I’m with you. I checked and online, I’m seeing that SF6 is 5 times heavier than air. Not sure how it gets into the upper atmosphere to trap heat. Maybe it is able to trap near the ground? Even the EPA’s website only has a very short blurb about its ability to trap infrared radiation. I’d be extremely disappointed to find out that our scientists are only measuring physical properties and not truly studying its affects on the climate.
The weight of a molecule states fairly little about how high into the atmosphere it will go.
Gases don’t tend to separate into well defined layers. They rather tend to mix even if there is no air movements to speak off.
A gas pocket that is heavier than the air can however linger around near the ground while it is dissolving into the air. An effect that can be seen around some volcanoes where the occasional release of more concentrated carbon dioxide can linger about in surrounding valleys for a while. (there is a few documented cases of this happening where whole villages has ended up suffocating over night.)
SF6 is so soupy that you can pour it like a liquid. Fans that move air will choke on it. Propane is notorious for collecting in low areas and it’s a lot lighter than SF6. So I wonder how bad it mixes and if it gets into the upper atmosphere where it can act as an IR reflector. We should find out before going to a different chemical, which could cause cancer or something (in California).
Pouring out CO2 over the floor will also have it linger about as it slowly dissipates into the air.
This is true for all gases that is heavier than the surrounding air. lighter gases will however float upwards while they dissolve. It is similar to throwing in a lump of metal into an acid, it won’t just all instantly go into solution.
Yes, SF6 is dense, but that doesn’t stop it from dissipating into the air.
The effect of gravity keeping heavier stuff towards the bottom of the atmosphere is a fairly weak force compared to the interactions within the gas itself. So even SF6 should be able to climb quite a distance into the air.
And I don’t think it needs to reach the upper atmosphere (50-80 km) for it to have a substantial greenhouse effect. H2O does a fairly good job at much lower altitudes (1-6 km for the majority of clouds).
What does “heavier than air” mean? A lot of things in the atmosphere (“air”) are “heavier than air”. Consider this question: why is there helium in the “air”? It’s “lighter than air”, right?
It doesn’t need to get very high – it’s a thermal blanket gas. Just like a regular blanket works when it is directly in contact with you. As long as it converts IR energy into molecular motion (heat) it prevents that IR energy from escaping into space. I think the comparison with CFCs getting to high altitudes is misleading as the effects of concern for SF6 aren’t altitude dependent.
Diffusion is a thing.
The gas doesn’t have to be in the upper layers in order to trap heat. Any presens of of an IR opaque gas at any level in the atmosphere will prevent heat from being radiated back into space.
I assume that this misconception stems from how the greenhouse effect is typically visualized, where rays are depicted as “reflecting” of the top layer.
(Or maybe how we’ve learned how CFCs break down ozone in the upper atmosphere?)
But, let’s for a moment assume that it needs to be in the upper layers of the atmosphere!
Despite SF6 being much denser than ‘air’, it will still mix. This is because fluids always mix, and gasses are fluids. The reason why is because above absolute zero, the molecules are under constant motion, which causes them to randomly bump into each other and completely mix. This process is known as molecular diffusion.
If you could somehow fill a bottle with SF6 at the bottom, and “air” at the top such that the gasses are completely stagnant. Due to diffusion, the gasses will still mix and form a homogeneous mixture.
In the atmosphere, we also have wind, which further aids the mixing process. So considering that SF6 has such a long half-life, it is certain that it has time to be transported to the upper layers of the atmosphere.
But, like I said, it doesn’t matter where the gas is located in the atmosphere. It still has the same heat capturing ability.
I hope that helps clarify things :)
Seems to me Ren, the point of the article is to draw attention to SWOT in our overlapping technical fields for which hackaday is increasingly recognised, eg on subject of SF6 usage and consequences of leakage in its proven GHG property ie Infra Red light absorbance/emission (part of GHG definition) exceeding that of the well known gases most often under discussion. I didn’t know the troubling scale of SF6 retention or magnification of its property as a GHG over that of methane, carbon dioxide and increasing levels of N2O from higher nitrate usage ie as some crops in decline, plus the looming threat of higher water vapour from all so called green hydrogen increase whilst CO2 still increasing ie a further double whammy whilst we transition to electric etc.
IOW. The post is a starting point with useful information, so thanks to hackaday for publishing this :-)
This SF6 data is available on the net though many not compelled to research so it’s good in my case it’s turned up as I have frequent debates with numb skulls over GHG properties especially those who can’t do basic arithmetic whilst ignoring spectroscopy even from Svante Arrhenius 120+ years ago – maybe declining cognition and social ethics an artefact of the ace2 neural receptor challenged by covid & it’s incessant mutations…
https://www.researchgate.net/figure/Normal-modes-of-vibrations-for-SF6-The-wavenumber-period-degeneracy-and-activity-of_fig4_259325378
It’s not as if we need spoon feeding, curious why you Ren, didn’t find something relevant and post it if at least to augment instead of not so subtle complaint, yes Ren you can argue, waste people’s time, draw attention to yourself Or get with the program, augment, dialectic critique at least with some engagement to offer something useful beyond one liner complaint :P
My lovely new particle accelerator contains 900+ kg of SF6. We’ll be careful not to let any of it out…
I personally don’t work with these high voltage and high power applications, but I can see the technical issues of “just switching” a few tens to hundreds of kV with a few hundred or more amps behind it.
Reading a bit onto the subject of Gas-insulated Switchgear, where SF6 is used, it seems its main advantage is its higher power density and this is useful if the substation is of a limited size. But for areas where room is less of a problem, then maybe less dense switchgear should be used.
Also seems like some GIS systems use CO2 instead, likely with some drawbacks, mainly that it has about 1/3 of the breakdown voltage in comparison to SF6. So the contacts must move away much further before the arc can’t reignite. (I for a bit wondered why argon weren’t used, but it’s breakdown voltage is even worse, by a lot…) SF6 also seems to have higher thermal mass than CO2, so this likely also helps quenching any arcs.
Though, one could ask about other arc quenching methods.
Like some circuit breakers/fuses have a set of copper plates that the arc is encouraged to jump between, but the distance of taking that route is significantly longer than jumping to the other contact, and the thermal mass of the copper also helps in quenching the arc. But I don’t know if this is practical in higher power applications.
Likewise one could perhaps make a more staggered switch, where in parallel to the main current carrying one, one can have a set of smaller switches with series resistors of increasing value. So that over a span of a few tens/hundreds of ms we can progressively increase the resistance of the link until it is open. This provides the current with an alternate slightly higher resistance route while keeping the voltages over a given switch bellow the voltage needed for the arch to form. The resistors will however have to dissipate a fair bit of power during the short period where they get to carry the current. I can though see legitimate reasons for this approach being a bit cost ineffective.
so never opening a high-energy contactor without a resistive bypass already closed?
The idea on the end about having a smaller bank of switches where we progressively step up the resistance is mainly to provide the current and all the inductive kickback a place to go.
The additional switches are likely always connected. And to be fair, a real implementation might have all these switches as the same thing. Just a large connector with a row of springy contacts that have a bit of a staircase shape to them so that one teeth at a time disengages. Where each of these teeth has a series resistance to the prior teeth in the chain.
Then it would be fairly trivial to make a switch with tens of secondary follow up switches that progressively increase the resistance over our switch while always maintaining contact till the very last switch pulls away. And by that point there won’t be any large currents forcing an arc to form.
Might still see a bit of corona discharge on the last switch as it moves away and gets sufficient distance to not break down the air. But the series resistance to this switch would be fairly high so an arc wouldn’t be sustainable. And yes, the last few teeth would need to rather quickly jump up in resistance, but the resistors would likely follow some exponential curve. By the time the last teeth is reached, it might be well into the hundreds of mega ohm range if measuring from that teeth to the primary connector.
A hypothetical resistor arangement might just be to start with 0.1 ohm, then 0.1 ohm for the next resistor followed by 0.2, 0.4, 1.2, 3, 5, 10, 30, 50, etc… (with this pattern we reach a total of 100 ohm by the 10th teeth, and increase one order of magnitude for every 3 additional teeth. This might however be too fast.)
Another advantage to this progressive change in resistance is that it would also work when closing the contact. And technically should work with DC voltages too.
The main reason SF6 is used in the first place is not so much for its dielectric capabilities but more for its quench.
Even rapid mechanical switchgear suffers from horrid contact degradation during close and somewhat to a lesser degree when opening. Increases in contractor speed can mitigate this somewhat, but in the end an inert gas that can sink some of the arc heat, displace oxygen and provide a stronger dielectric significantly increase the service life of these devices.
Inevitably solid state switching is the only real option to completely displace dielectric gas. Fortunately such devices are becoming more and more feasible and more cost effective year on year. Nowadays it’s quite reasonable to control tens of KAmps at thousands of volts AC using large button SCR banks. IGBT’s are slowly gaining ground in the DC space as well.
Arc quenching is a useful feature to have if one’s switch creates an arc.
But the hypothetical switch stated above wouldn’t create an arc in the first place.
Negating any need for SF6 in such a switch.
But I can however see downsides with the concept of having multiple switches used for progressively changing the effective resistance over the switch. Mainly that it would open a bit slower, and likewise take a bit longer to close. (However, the idea of using such a switch is mainly for day to day operation, reacting on faults should likely use a more sacrificial breaker.)
In the end, the idea of progressively changing the resistance of the switch is that it becomes the mechanical equivalent to a solid state switch.
Anyone who has fiddled with power electronics and turned off a FET too fast will know that if the current doesn’t have anywhere to go, even parasitic inductance can kill the FET with ease. So turn it off “slowly” as to gradually let the current diminish in a controlled fashion.
I am a switchgear engineer. I own and operate a power equipment business. We maintain, install, and upgrade gear up to 500kv. I do believe it’s correct that the power industry is using the largest amount of sf6 however the article seems to allude that there is a massive amount of leakage in these breaker and switching bottles. While I’m sure there is some leakage it is near negligible I would imagine as the destruction that occurs when a bottle is void of sf6 and has allowed atmosphere air in is quite impressive. If every bottle of every breaker or switch we have installed leaked at the rate being alluded to there would be multiple large arc flash incidents every single day in every city and every substation.
Don’t get me wrong these bottles do leak sometimes and there are incidents because of it. We as a power industry all push for any owner of a bottle operated system to have these breakers and switches maintained on an annual basis if we cannot convince them to quarterly maintenance. My primary injection test kit will find a bottle that has lost less than 10% of it’s sf6 very easily. We then remove that breaker from service, the bottles will then be emptied completely into the recovery tank and new or refurbished bottles will be installed. The industry as a whole takes sf6 leakage very seriously, not because of the atmosphere but because of the destructive nature of a large current high voltage arc.
Near the end of the article it mentions utilizing a vacuum instead of sf6. While this works on paper, in practice vacuum is more difficult to seal and maintain than pressure. As well as the issue of bottle maintenance, I am unsure how I would test the bottles for vacuum the same way I test for pressure. It would be a large undertaking for the industry to switch, not impossible but very difficult to make. In my opinion moving to a different inert gas would be the better choice. However I do not know or have a suggestion of one that would be sufficient.
One of our customers wanted to use vaccum sf6 breakers… This joke still goes in our office
Maybe the customer used “SF6” to refer to GIS breakers?
Like some electricians calls all multimeters a “Fluke”.
Or how some people call all electric cars a “Tesla”.
Or all tablets an “Ipad”. Or “Iphone” for smartphones.
Or how people say that they want “ESD work surfaces” when they in fact want the opposite.
One can likely make a long list of common things that people say but either mean something completely different, or something a lot more general than technically specified.
Though, sometimes these types of blunders in communication can lead to all sorts of annoying problems.
Actually, vacuum interrupted, SF6 insulated breakers are very, very, common. It will be a piece of switchgear that uses vacuum interrupters housed inside an SF6 filled enclosure. In this case the gas is being used as an insulating medium for BIL purposes rather than as the interruption medium – which is done in vacuum. In the past I worked for a manufacturer who specialized in making exactly this type of equipment. These days I work for a manufacturer who designs and makes SF6 free switchgear using vacuum interrupters and solid dielectric insulation – this technology has now largely superseded SF6 in lower voltage classes (up to 38kV) – but at higher voltages SF6 still seems necessary for the time being.
Thanks for your expert perspective. Seems crazy that anything with fluorine in it can be famous for its stability but here we are 🤣 Are there any signs the industry considers SF6 sub-optimal or problematic and is trying to come up with a better solution? Or is it a good enough local maximum, at least without external changes?
Fluorine compounds are in general quite stable, because the fluorine has already reacted. For example, teflon and other PFOAs, as well as nitrogen trifluoride. Obvious exceptions include hydrofluoric acid, and complexes with other electronegative elements e.g; halogens.
Vacuum is the opposite of pressure. If you can’t ‘keep in’ a vacuum, it means you can’t keep in a pressure either. Of course it’s harder to keep a vacuum ‘in’, because most molecules in the atmosphere are smaller than SF6. But if you keep dismissing vacuum because it’s ‘just harder to maintain’, then it means you’re dismissing a whole lot of research that could have been done, which would have benefitted the ‘keeping a vacuum in’.
In the end, a vacuum is nothing more than ‘keeping out molecules’, which is the same as ‘keeping in a pressure’. The pressure is just 0.
The sealing is what it’s about. And I think that research on sealing is on a low burner, because the industry only needs seals that are ‘good enough’. I.e. seals that can keep SF6 in, instead of seals that keep every other molecule out.
I know it’s harder to keep every other molecule out. Just saying that I’m sure that our industrial efforts in that direction are very low, because we already have stuff that works for us.
Even if you magic up a solution that remove 100% any ingress from outside air (which is impossible) you still have to worry about outgassing of the materials in your vacuum chamber – and basically everything out gasses at low pressure to some extent or other…
The only way to maintain a real vacuum is constant pumping, if a very poor vacuum is enough to prevent arcing maybe its possible to make it workable as keeping only a little under atmospheric pressure isn’t particularly hard, and every month/year – whatever the service interval they can go round and spend a day or two pumping it all the way back down again.
Vacuum tubes stay pretty darned empty for decades with no active pumping, just a getter. With wires going through the envelope. It may be a pain. It may not be feasible at all for switchgear. But it’s not as simple as it being categorically impossible for a passive structure to maintain high vacuum.
Nothing can maintain it indefinitely, not that I disagree you can make things that hold relatively good vacuum for a very good time – the key to that however tends to be one part glass evelopes you never ever open after sealing, with no serviceable parts inside, which I don’t think really works as a design for HV switching – and even those valves and lightbulbs etc do leak, its just slow.
Thanks for your insight !
Having built quite a bit of x-ray high power equipment – would rather avoid vacuum insulated HV gear. Anything that can spontaneously turn into a dangerous x-ray source should be well avoided.
But sometimes vacuum and HV are unavoidable. The present pulsed power machine that’s being worked on will have very high voltage and vacuum around the load section. Pulse power machines though, often tend to be designed to generate radiation, so we all know to keep out the way and hide behind adequate shielding.
I didn’t see any allusions to a specific leakage rate. And I would suspect that most of the “leakage” is at the end of life for one or another piece of equipment. Either shortly after the equipment leaves service, or shortly before…
But in the end, there’s no question that roughly 100 percent of the SF6 that you manufacture *will* end up in the air unless you actively disassemble the molecule. There’s noplace else for it to go. If it has a lifetime of 3200 years, or even a half-life of 3200 years, then the time it’s going to spend inside your equipment is clearly going to be a lot shorter than the time it spends outside. How are you going to contain and recycle the same gas for three millennia?
There’s a thing that I don’t understand: the air density is about 1,2 kg/m³, while the SF6 density is about 6,17kg/m³. Being so much more dense, it should stay at very low altitude and, thus, not have so much effect… Or am I missing something?
The fun thing about gases is that they mix, or to a degree dissolve into each other.
Similarly, lead iodide is also fairly heavy but will dissolve in far lighter water. It doesn’t really settle at the bottom, it will be an even solution throughout if left long enough. It doesn’t really matter how tall the beaker is.
Gases however mix even more easily. It is just free molecules flying about the place randomly. If another molecule comes in that is a few times more heavy doesn’t really matter, it will bounce about like any other particle.
Gravity only starts to matter when one reaches rather excessive altitudes. SF6 shouldn’t have much issue getting up a few km. (and that is all that is needed for it to have its substantial impact.)
https://en.wikipedia.org/wiki/Diffusion
Since it’s a molecule that more easily converts IR energy (photons) into thermal energy (heat) it interferes with the radiation of heat from the ground and oceans back into space. It doesn’t matter what altitude it is occupying, it contributes to warming the atmosphere.
Really huls, what weird line of science/physics are you on, what is the provenance of your vague ideas ????
Pray tell Why you appear to deny Spectroscopy ???
Why pepper this forum with unsupportable claims – FFS it’s Not basic physics !!!
Gases mix at the drop of a hat, it is what they do really really really well. Expecting gases to separate into layers would be a bit like expecting water, oil and mercury to separate into layers while being constantly shaken.
You can lower the temperature and slightly increase the minuscule gravitational gradient, which is used to separate isotopes in gas centrifuges. But they operate under near ideal steady state conditions with only one gas! Gases in the atmosphere are never in steady state, on a planet that undergoes a 24 hour heating, cooling cycle.
There is the exact same concentration of helium and hydrogen at ground level as there is near the vacuum of space. Gasses mix and mix really really really well.
Honestly, if we aren’t banning it’s use then we should at least make a crazy high tax for items that use it in order to dissuade it’s use.
Right and replace it with what? Vacuum switchgear that is much more expensive and less reliable?
SF6 doesn’t even compare to CO2 and methane in terms of released quantities.
A more reasonable approach would be monitoring and fixing leaks, combined with active recovery during maintenance (much like refrigerants) assuming that’s not already standard practice.
“Right and replace it with what? Vacuum switchgear that is much more expensive and less reliable?”
Why not? Nothing in life is free, you pay for everything one way or another. SF6 *seems* cheaper, but only because the bill is split between 7.1 billion people. With vacuum switchgear, the bill is split between the people who benefit from the switch gear.
To be honest, the second option seems a whole lot more fair towards those 7.1 billion people. And in the long run is also more fair to those few million that benefit from the SF6-filled switchgear, because they and all their future generations have to live on this Earth as well.
But well, there’s always Darwin. While the Earths atmosphere changes, some people’s genetic strains will be able to adapt, while others won’t. So there is always a good chance that your offspring will be able to adapt before your genetic line goes extinct. Maybe the odds are good enough to safely bet your future offspring on?
If you take that argument so far you might as well go round smashing all machinery of any sort or murdering folks for no reason at all beyond they breath out and it would be so much better if they didn’t…
Practicality means there will always be gasses with some green house effect in use, its not even remotely possible, or desirable not to have them – as everything on Earth that respires and many things that don’t create and/or consume them, plus complete absence would make the planet too cold for life remotely as we know it…
Its responsible use that matters, and SF6 used properly isn’t a major problem – far better than the alternatives I would suggest – a well built system shouldn’t leak even over timescales getting on for forever, where using something like a vacuum system you will be burning lots of energy maintaining that vacuum – which makes sense for systems that are not running critical tasks to modern life 24/7, like your HV lab setting perhaps, but not the switch gear for the grid we all rely on quite heavily. Maybe CO2 has enough merit to be worth using, but its still a green house gas, and all the extra materials needed in the switch gear to make it larger so CO2 is possible have environmental costs too.
“A more reasonable approach would be monitoring and fixing leaks, combined with active recovery during maintenance (much like refrigerants) assuming that’s not already standard practice.”
Also, this goes just as much for vacuum switchgear. Less reliable and more expensive…
I bet they are not less reliable. But they are more expensive. And the sole reason for that is that they need more maintenance.
So, if you’re advocating to keep SF6 and increase the monitoring and maintenance effort, you’re making exactly the same case as for using a vacuum and increasing the monitoring and maintenance effort.
Bottom line is that you want to increase the monitoring and maintenance effort with a minimum, still trying to minimise cost and pushing the problem forward towards next generations so that you don’t have to deal with it.
Crazy high taxes just get passed on to the end user, meaning that we’ll still use about the same quantity of bad stuff in power delivery systems – and even more over time in response to population growth – but the poorest among us won’t be able to afford to buy power. Making poor people suffer more is the opposite of progress.
If we’re saving the world, we have to save it for everybody.
Hang on a minute. Yes, per-pound this stuff is much worse then CO2/Methane, but how much is actually being released? How much of the overall impact does it make up?
I can see not releasing it en-mass during manufacturing is a worthy goal, but how much of this dense gas is really going to leak out of heavy switchgear over it’s operational life?
I doubt enough to justify the fear mongering.
I’m not seeing much in terms of fear-mongering here. It’s something to take note of, and it’s a good thing the early insights (the general drift of this article is also found in publications of the 90s already) are being taken seriously.
Now consider two things:
1) production is increasing, not decreasing
2) on the timescales SF6 persists, the mere circumstance that it’s being used means all of it is either reacted or released. All of it. If 10% leakage in a decade is typical for an industry, this means the half-life for leakage is a short 6.5 decades.
The good news is, there’s absolutely no need for rushed decisions, but we definitely should figure out how to replace SF6 with something else.
From the article:
Taking into account the much higher GWP (22800) of SF6, this makes its 2018 emissions equivalent to about 205,200 Gg, or 0.6% of annual CO2 emissions.
Yes of current emissions, but if we are really doing to head towards net zero, hopefully the emissions will go down by a factor of 10? or more, and the SF6 proportion will then rise if not also checked.
Just because the proportion rises doesn’t mean more damage is being done. And, if anyone ever does find a viable alternative, SF6 usage will of course shrink.
Sometimes “This is a bad solution” is overridden by “but there’s no better solution available”.
Without SF6, you see a dramatic reduction in viable power delivery.
And, at current Western population-per-area densities, it doesn’t take much reduction in power delivery before people start dying. Sure, global warming might (eventually) kill some people, but turning off the power grid kills a lot more people, and does it a lot more quickly.
The problem isn’t “making sure we eliminate SF6”. It’s “developing something that can let us migrate off of SF6 without killing a lot of people”. In other words, it’s still in the realm of engineering rather than politics.
Oh really huls, more crap & uneducated tripe, repeating flakey useless propaganda Not an Educated position !
Claims are cheap, anyone can drop changers drawing attention to themselves, education so Very Important !
CO2 is a waste product from animal life, plants & some bacterial species exploit this waste product for growth mostly carbs.
The molecule of life is clearly water With the moderation and complexity offered by multiple minerals…
To push an uneducated claim of “physically impossible” re CO2 is a Huge failure of education huls !
Where the fark did such a stupid claim arise from And why didn’t you check it ???
Please learn the essential irrefutable physics of:-
1. Spectroscopy
2. Radiative transfer
3. Statistical mechanics
Add to that
4. Psychrometry
5. Beer Lambert quantification (caution some calculus)
6. Isotopic signature
Think man (huls) ExxonMobil fully conceded AGW back in 1982, shortly thereafter affirmed by Shell & BP with ExxonMobil’s climate model, within good error bars, accurate to this day even after 39 years FFS !
Svante Arrhenius was the most notable who warned of this GHG issue 120 years ago.
Physics works, you can do the arithmetic yourself. Where did you miss spectroscopy in school ???
Perhaps you are misled to imagine an IR photon causes all gases to convect, do the arithmetic, it’s called momentum transfer.
Yes I know it “seems” hard but it’s really quite simple, especially when you appreciate gases have varying angular momentum in the atmosphere in 3 axes at the very same time whilst multiple collisions with other GHGs etc especially water vapour.
Satellite studies eg RSS fully confirm the GHG effect And at multiple wavelengths, higher surface temperatures whilst less IR leaving to space ie higher IR resistivity.
Please don’t embarass yourself despite hiding behind anonymity, where the heck did you learn highschool physics ???
Take it one step at a time just What is Physically impossible ???
Leaks are much easier to seal against. SF6 is a fairly bulky molecule so won’t diffuse as quickly through an o-ring or membrane as air would trying to get into a high vacuum. The impact of the (relatively) small quantities of SF6 used in switchgear can be mitigated using better pressure engineering (more expensive but probably not as much as getting rid of SF6) and leak detectors. I think I’ve seen an SF6 leak detector in my travels, can’t recall.
It will diffuse into the upper atmosphere eventually. It’ll spread out and sink initially, but it’ll get there eventually. It’s a rate process. Someone also said that there’s ‘helium in air’? Not much, it escapes very easily, one of the reasons why it’s a shrinking and increasingly expensive non-renewable resource.
CO2 is not a great greenhouse gas. In terms of absorptivity in the IR it’s pretty average compared to most anthropogenic gasses (e.g. NOx and the big one nobody wants to talk about because it’s too complicated – water vapour) There are much worse ones. But it’s a convenient metric. The comment earlier about molecular cross section and degrees of movement is a good one. More stretches – broader absorption generally.
You can see the questions here posed by the engineers – never send an engineer to do a scientist’s job, because they will be absolutely certain that they can ;P
Sounds like a good gas to release on Mars to
increase the surface temperature.
SF6 is used for convenience and cost, for the very highest voltages nothing beats pure size. The lower voltages will just have to start making do with bigger equipment too.
Exactly, just ban it. We banned CFC’s as refrigerants. We had switchgear around long before SF6 was used for this.
CFC finally got banned (oh so successfully, not…) after a prolonged period with proof of the harm they were doing, so far SF6 doesn’t show enough problems to be of such concern – its certainly a potential problem to keep an eye on, but with the litany of existing major problems we are as a species doing very little to fix (or at it stands in general still making some of them worse)…
Those problems really need to take priority, and as it stands using SF6 is actually useful enough in making the electric stay on as we move to a more renewable powered future, if/when it becomes a problem big enough to register hopefully it will get dealt with more effectively than the CFC ban, there are certainly other switchgear options, but for now it seems rather worthwhile…
Let’s walk down this road of first-order thinking:
We ‘just ban’ SF6 right now, today.
MAXIMUM SCENARIO:
The power delivery services do what they must, and take down all the SF6-filled equipment.
Because we banned SF6 without having an alternative, we no longer have the ability to deliver power.
Electric cars, putative saviors of the world, can’t charge. Electric trains don’t run. Aircraft can’t fly because we can’t produce fuels, electronics, plastics; we can’t run computers, radio, radar, even illuminated beacons and runway lights.
Winter reminds us what cold is; we build fires of natural gas or oil or wood or coal everywhere to survive, vastly increasing all combustion pollutants in the atmosphere and sharply increasing land damage from renewed fossil- and living-fuel extraction. Many hospitals close because they can’t work effectively without reliable light and temperature control.
Summer reminds us what hot is; we first suffer and eventually migrate en masse. It gets crowded and violent in the remaining livable regions, a war for being, survival of the fittest.
The entire world is a third-world country, we slide toward a new Dark Age.
This is the first few years after we lose power.
MINIMUM SCENARIO:
The power delivery services negotiate or cheat the takedown edict and remove SF6-filled equipment as quickly as they can roll back to earlier, less reliable solutions.
We still have power, but reliability falls off sharply. Interruptions increase. Costs spike, too, because somebody has to pay for the refit, and that cost is passed down to us, the consumers; the already poorest third of the population slide toward utter destitution. The middle class thins and falters. The rich do ok, because they’re rich.
Densly populated regions, hospitals, factories, businesses of all kinds install comparatively high-pollution diesel generators because solar+battery solutions aren’t reliable enough, available enough, or affordable enough, and local wind power is too dependant on, well, the good fortune of wind. Pollution spikes, smog returns to major cities.
The older, less reliable power delivery solutions include catastrophic failure modes, sparking increasing numbers of wildfires – or what California currently calls “just another day of the week” – driving massive destruction and pollution events.
I love how you act like our only option is to watch law making corporations hold people in poverty.
If only someone could do something.
Good article that sheds light on an important and underreported topic.
However, the article’s implied resignation that SF6, despite its growing environmental impact, will continue to be part of energy infrastructure into the future is misplaced. There has recently been an acceleration of sustainable innovation in this space, and will continue to be as regulators in the US and EU scrutinize SF6’s use in switchgear more and more.
So much so that OEMs recently released a statement underlining the need for, and their commitment to, a transition away from using SF6 in switchgear:
https://www.nuventura.com/post/toward-t-d-equipment-free-of-fluorinated-gases-for-sustainable-climate-neutral-power-grids
Wow. I’ll you people that rode into the comments and screamed that we “should just ban it”, should go spend some time learning about Chesterton’s fence.
We are not going to save the planet when people keep coming up with caveats.
If the consequences of releasing any SF6 into the atmosphere was death to the person doing it. things would rapidly change to make releasing it a zero possible option.
Now you an roll back from that extreme viewpoint, but precautions of that level take place in virology labs (maybe not in China).
If we are killing the planet by releasing all these gases and there are in effect zero consequences for each individual release, the planet is going to die from cumulative effects.
So sure, find some middle ground, but releasing harmful chemicals into the atmosphere has been for decades part of acceptable business practices.
If this was stopped and business would go out of business and the board members should go to prison, rapidly we would have saved the planet.
Methane is a greenhouse gas. Farts contain methane. All people fart. Therefor all people should be in prison.
The planet won’t die. The people on the planet will die, with some of the other plants and animals as bycatch. We’re the problem; nature is in the process of sorting us out.
Whether that’s good or bad, I leave to to the philosophers.
That “quenched emperor” banner art is superb!
Someone got it!
Yes, it’s another great original art one – good work, Joe Kim!
“some hope can be gained from the way the world came together to banish CFCs”
Banned CFCs traced to China say scientists – 22 May 2019
Further detective work in China by the Environmental Investigation Agency in 2018 seemed to indicate that the country was indeed the source. They found that the illegal chemical was used in the majority of the polyurethane insulation produced by firms they contacted.
One seller of CFC-11 estimated that 70% of China’s domestic sales used the illegal gas. The reason was quite simple – CFC-11 is better quality and much cheaper than the alternatives.
This new paper seems to confirm beyond any reasonable doubt that some 40-60% of the increase in emissions is coming from provinces in eastern China.
Fun demo of the SF6 density: https://www.youtube.com/watch?v=iQWtZd8jM3g#t=1m
I think you really need to look at the magnitude and whether this is the best problem to be working on. If the issue is the UV absorbed by the gas, I would ask whether painting all of our transformers and outdoor switch gear dark colors not to mention every asphalt paved area is absorbing more UV than this gas. It does not take much to conclude that all of these black parking lots and streets are absorbing tons of heat and warming things up. Anyone living in a city can tell you about that. There are trade offs however, here in Chicago there was lots of talk about light colored roofs lowering air conditioning bills however they do lead to higher heating bills in winter (which is mostly done with natural gas here). In this area of the country we cool with electric (a lot of it nuclear) and heat with fossil fuels. It probably makes more environmental sense here to work on the heating side of the equation rather than the cooling side of the equation.
One thing I have not seen addressed at all is that while lowering usage of fuel for vehicles is a good idea environmentally, where then do we get the natural gas and propane that is used to heat a large area of this country. Natural gas is normally found while drilling for oil and propane is a byproduct of refining oil. Please don’t tell me that electric heat is the answer, the economics of that do not work here in the northern areas of the country. Going to electric vehicles and electric heat for all of our buildings will not even be close to feasible unless we get very cheap plentiful nuclear power (and greatly upgraded infrastructure, most 100 Amp service in this city will not even support heating a building). I don’t think solar and/or wind will come close to the energy density required for all of these changes to electric.
I have yet to here any politician talk about heating, it is always about cooling and vehicles. Heating is the elephant in the room that no one wants to address. If someone really wants to impress me, please explain how a renewable energy source is going to heat our buildings in major northern cities.
unless we get very cheap plentiful nuclear power…
Good thing that already happened.
Some fluids will separate even if forcibly mixed, e.g. oil and water.
There is almost no helium in the air. Helium and hydrogen tend to float right to the top of the atmosphere and eventually lost into space. Commercial helium comes from natural gas where it gets trapped after being created by alpha decay from radioactive elements.
If anyone is interested: SF6 is also used in ophthalmic surgery. However the amounts are likely miniscule in comparison to its industrial use
It might be just a stupid idea , but i’ll post it anyway : just put a ban on any new production of SF6 , and not on the use of it. So how do we make new switchgear ,would you say?
Just recycle it from the ambient air. Due to the density of it , most of it will be close to the earth’s surface , and there must be a way to harvest it.
It will be more expensive – of coarse – but everything comes with a price as said before.
Every SF6 molecule that is produced , will eventually end up in the atmosphere ,and stay there forever – well, 3200 years – so knowing this ,its just plain stupid producing ever more of this substance ,while there is plenty of it just floating around ,and doing bad things for our climate.
All SF6 which is in the atmosphere at this very moment has already leaked out of something, somewhere , being at production sites ,scrapyards or accidents.
I think that is probably a solution for the future, once there is somewhere around of enough of it in circulation – do it now and you just cripple the electricity infrastructure projects that really are needed and by all current evidence not leaking meaningful amounts – so most of it will get pumped out and then back in again without harvesting from the air…