Here at Hackaday, we love projects that result in useful lab equipment for a fraction of the cost of professional gear. [Lorenz], over at Advanced Tinkering, built his own instrument for Laser-Induced Breakdown Spectroscopy, or LIBS, and it’s quite an impressive device. LIBS is a technique for analyzing substances to find their chemical composition. Basically, the idea is to zap a sample with a powerful laser, then look at the little cloud of plasma that results and measure the wavelengths emitted by it.
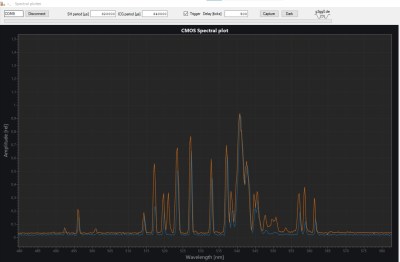
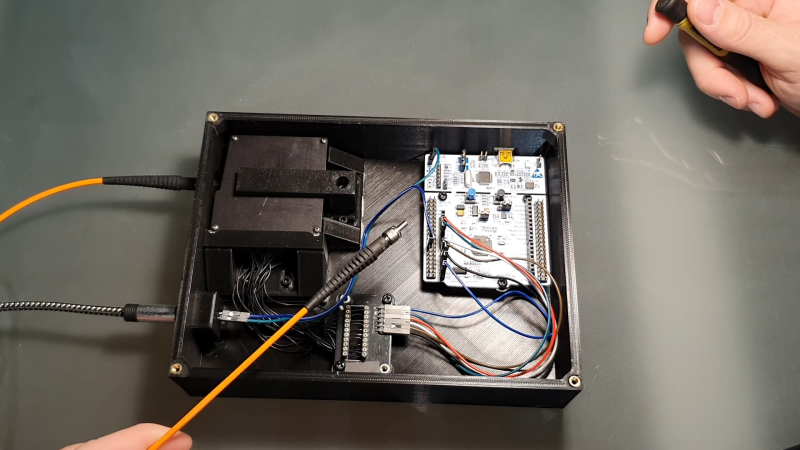
The laser [Lorenz] used is a Nd:YAG unit salvaged from a tattoo removal machine. After it fires a pulse, a photodiode detects the light and triggers a spectrometer, which consists of a diffraction grating, a few lenses and mirrors, and a linear CCD sensor. The grating splits the incoming lights into its constituent components, which fall onto the CCD and trigger its pixels. An STM32 Nucleo board reads out the results and sends them to a PC for further processing.
That processing bit turned out to be a full project on its own. [Lorenz] called upon [g3gg0], who software that simplifies the operation of the spectrometer. First, it helps with the instrument’s calibration. Point the detector at a well-known light source like a laser or a fluorescent lamp, then select the expected wavelengths on the resulting spectral plot. The software then automatically calculates the correct coefficients to map each pixel to a specific wavelength.
The software also contains a database of spectra corresponding to chemical elements: once you’ve taken a spectrum of an unknown sample, you can overlay these onto the resulting plot and try to find a match. The resulting system seems to work quite well. Samples of iron oxide and silver oxide gave a reasonable match to their constituent components.
We’ve seen other types of spectrometers before: if you simply want to characterize a light source, check out this Raspberry Pi-based model. If you’re interested in chemical analysis you might also want to look at this open-source Raman spectrometer.















This is an awesome diy project! The possibility of having one of these spectrometers in a community accessible lab associated with a local school or public library is very exciting. Looking forward to learning more about the range of chemical analysis that is possible.
Would a 10W 405nm laser or 40W CO2 laser + raspberry pi camera spectrometer work for this?
The laser he’s using puts out pulses of 100 megawatts to produce the plasma. Entirely different class.
So you’re saying to horrendously overdrive it and use a 10,000:1 duty cycle right?
The type of laser usually used is a Q-switched laser that allows the laser medium to absorb energy over a period of time (microseconds) until the Q-switch saturates then suddenly all the energy in a matter of nanoseconds. The instantaneous power is often like a gigawatt but since the time is so short the total energy is relatively small. The tank laser I used for years only put out about 20 millijoules. The one mentioned in this video is capable of 20 mj to 2 joules. Now that’s serious overkill. I have never operated mine over 500 mj for fear of harming it 0or something else.
Ooooo, I’ve had people hawking portable LIBS guns at me and they’re always like $50k plus. But just being able to identify metal scrap in the makerspace isn’t worth our entire annual budget.
A DIY option, on the other hand…
There are many ways to identify metal but I would caution you that this may not be a proverbial silver bullet because it only measures the surface which means coated metals may not register as their true content. This can be overcome by cutting the metal and testing both the inside and the surface to tell you the kind of metal and what (if anything) it’s coated with.
Given that most scraps already have a cut edge visible, that doesn’t sound like a bother at all, but thank you for the heads-up!
LIBS allows one to build a depth profile of a sample by repeated pulses so no need to cut the sample.
Could you detect contamination in water with this?
there is no single analytical process which will detect all forms of contamination. i doubt this would do anything useful in that situation.
contact a competent water quality lab.
If I replaced the laser with a telescope could I use this for stellar spectroscopy?
Short answer, no.
Due to beam divergence, when it reaches the star, it’s intensity will be too low to break down it’s material. And even if you succeed, the intensity of resulting spectrum would be below your detection threshold due to the stellar distances and the stars background light if it exists.
Star spectroscopy is based on either the fact that it is a sun etc., or is illuminated by one.
Unless you can send your instrument to the star and it can withstand the stars atmosphere it will probably not work.
“Unless you can send your instrument to the star and it can withstand the stars atmosphere it will probably not work.”
I don’t have that kind of money, so I guess it’s not, thanks.
Not to mention that the round trip time will be measured in years.
That is incredibly awesome. Please think about making this into an open source product. Think about analysing ones own saliva or blood in order to find out what stuff could be missing (or too much), and then simply eat accordingly. In my opinion, products like this will create a multi-billion size market soon.
Hello, is quantitative analysis of simple metalurgical samples within reason? Home iron smelters could benefit from carbon and other element analysis in samples to determine sutability for heat treating.
I have been working on a LIBS system for 9 years now. Started with a Abrams tank range finder laser (SSY1) and moved on to the same laser used here. My first spectrometer was a home built spectroscope. Been using a BW tech and an Ocean Optics USB2000 for several years. I purchased numerous elements to build an Atlas of LIBS Elements which is available at : https://archive.org/details/atlas-of-elements. I am interested in conversing with anyone interested in LIBS on an amateur basis.