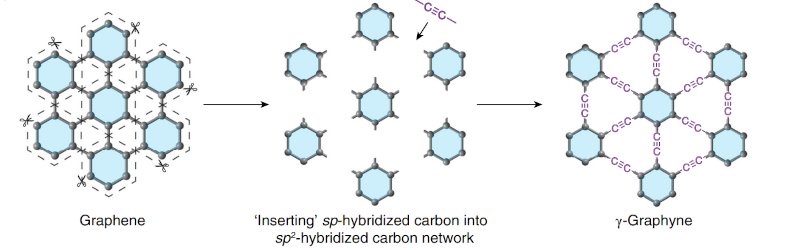
Before you jump down to the comments to chastise us for misspelling graphene, note that graphyne is similar to graphene but not the same. Like graphene, it is a two-dimensional structure of carbon. Unlike graphene, it contains double and triple bonds and does not always form hexagons. Scientists have postulated its existence for decades, but researchers at the University of Colorado Boulder have finally managed to pull it off. You can also download the paper if you want to wade through the details.
Carbon forms like fullerene and graphene are well-known and have many novel uses. Other allotropes of carbon include graphite and diamonds — certainly two things with wildly varying properties. Graphyne has conductivity similar to graphene but may also have other benefits.
The process is called alkyne metathesis, a fancy name for an organic reaction that reforms alkyne bonds. The process does require kinetic and thermodynamic control, including performing the reaction under argon. The entire produced is outlined in the paper under “Methods.” While it takes a little more than a test tube and a bunsen burner, it doesn’t sound like it takes anything too exotic — some chemicals, a Schlenk flask, liquid nitrogen, and a hot oil bath. This is something a well-stocked home lab might be able to pull off.
We still don’t know what to do with the graphene we make, but it isn’t that hard to make it. There are several different methods. Maybe we’ll see DIY graphyne soon.















This is just the beginning of an entirely new field of molecular construction. If you look at the work of Paul Harrison and you realise that the tiles can represent molecules then you can see how it is possible to program 2D structures at the molecular level, or even create Turing complete cascades of molecular interactions (see his other work for an example of that). https://logarithmic.net/ghostsurn/0.2/tile.html#bg=%23ffffff&outlines=1&rotation=1&grid=0&width=15&height=20&max_memory=100000&effort=250&scale=30&tile0=A3-3–&pal0=%23fce94f&weight0=1&tile1=-A33–&pal1=%23d9138a&weight1=1&tile2=a—a-&pal2=%2312a4d9&weight2=1
I’ve heard of the term “Turing complete” in context of programming, but how does it pertain to molecular interactions?
I assume the Turing completion is the same concept. Think of making a substance that is programmed to make a substance which could include making itself. Makes me think of the Tok’ra tunnels from Stargate SG1.
Ah the grey goo scenario…
https://en.wikipedia.org/wiki/Gray_goo
The “grey goo scenario” is a silly thing to worry about.
What grey goo fear advocates fail to realize is that it already happened. We are the grey goo. Us and all the other life on the planet. Evolution has had billions of years to come up with a self replicating nanobot that is capable of using all the available materials but it hasn’t. It has also had billions of years to train it’s “bots” to compete with one another.
I don’t think that a human being is going to accidentally engineer something that outcompetes all those natural molecular robots that have had billions of years to evolve ways to compete and defend themselves. Something that does environmental damage? Sure. But not a full on grey goo scenario.
I think what he’s basically saying is that we could perform computations via the interactions of molecules/computation at a molecular level.
The classical Turing Machine consists of a tape along which a reader head moves.
Ribosomes move along DNA and encode proteins.
It isn’t that much of a stretch to imagine a molecular Turing Machine.
It is explained very well here –> http://www.logarithmic.net/pfh/blog/01104447019
Talking about allotropes…
Just yesterday I read up a bit about Cubic Boron Nitride. It’s the second hardest material, and because it does not dissolve into steel like carbon (and thus diamond) does, it is used a lot for milling and turning hard steel alloys.
The hexagonal allotrope of Boron Nitride is apparently also used as a lubricant, just like graphite
https://en.wikipedia.org/wiki/Boron_nitride
Woah woah woah, diamond is soluble???
There is the formation of carbides at higher temperatures, e.g.:
https://cadem.com/why-diamond-tools-cannot-cut-steel/
Beyond that, carbon diffuses rather neatly in iron, forming various phases (google “iron carbon phase diagram”). The fun part is perhaps that it doesn’t matter that much which carbon allotrope in particular one starts with.
Yep. Steel really likes to absorb some carbon, especially when it’s hot such as during machining operations.
Diamonds can burn too, even though they do not release enough energy to sustain their own combustion, they do get burnt in fires.
Diamond is hard, but that does not mean it is impeccable marvelous indestructible material that the jewelry industry lobby wants us to believe it is.
After it became quite common and relatively simple to make diamond that same jewelry industry even convinced the “general public” that “synthetic diamonds” are not “real diamonds”. They’ve got an enormous lobbying machine to protect their own air castle, and have been successfully planting fake news for 50+ or so years. Jewelry industry is a dirty business.
You can diamond in about any quantity you want The stuff costs around EUR 300 per kg, the only reason “gem quality” diamonds are expensive is because of artificial scarcity caused by the diamond industry, which is (or at least has been for 50+ years) dominated by a monopoly from a single company.
So there has been a race going on, if you can make an “artificial diamond” which is indistinguishable from a “gem quality” diamond, you can get rich because of the ridiculous price difference, and the jewelry industry has been forced to ever keep inventing new tests to be able to find any difference to save their multi billion industry branch. One of the “distinguishing” marks is that homebrew diamonds are more pure than natural diamonds. and the tests involve the detection of trace amounts of impurities. (and the people who want to get rich by making diamonds are trying to figure out how to add those impurities)
Just to be clear: the EUR 300/kg diamond is “industrial quality”, it’s usually not clear, nor fit for jewelry. It takes more effort to make mono crystalline transparent diamonds, and those are probably more expensive.
Flammable too, in cryogenic oxygen of all things. Easily goggled.
A friend runs a machine/welding shop and has a rather large surfacing machine. He had to use CBN inserts to flatten engine blocks, etc. Regular carbide dulled too fast, and diamond does as paulvdh notes. Jim complained bitterly about the cost–back then it was $200 for one insert. He was eternally grateful when I found a pack of 10 for $40 (on eBay!).
I’m very curious about this.
What’s the quality of those affordable chinese CBN insert compared to those other ones?
As a hobby-machinist it’s not likely I would wear them out. Breaking them though unintended abuse is a real possibility though. My main use would indeed be to machine a ballscrew as ANdy Pugh below notes. But it’s not really mandatory. Once the outer two or so millimeters are ground off they are decently machinable with regular carbide tipped tools.
I also guess that a broken CBN tip can still be used nicely to dress grinding stones.
If you want to machine your own ballscrew ends then getting some CBN inserts from eBay is a good way to go.
[url]https://youtu.be/SmuZXXP_hMY[/url]
So, we went from gray goo to yellow goo?
Oh…
I copy and pasted your link into DuckDuckGo (including the URL brackets) and it took me to a Japanese video which seem to show mixing a mango with yogurt made a yellow goo.
Without the URL brackets, it took me to a video of a lathe…
Well, from what I’ve heard a couple of labs are closer to having a good synthesis method for actual 2D graphyne sheets. All this is is an expensive and inefficient method to make chunks of maybe graphyne layers. I’m guessing they wanted to publish quickly to get their names on it before good methods come out. I’m not an expert myself though, I guess we’ll see.
Does anyone else feel like Nature is almost just a tabloid full of sensationalist headlines at this point? Not it still has good stuff sometimes but most of it is not as well vetted as it should be, just gets clicks.
Graphyne is super cool though. A lot of progress to look forward to for sure.
We always hear a couple labs are really close to something. How long has it been since someone was on the verge of perfecting a roll to roll graphene synthesis that would be so good it’d be done commercially? 10 years?
With all those triple bonds and one-and-a-half bonds in the aromatic rings, that’s going to be a rather energetic structure. Might be suitable as a fuel or as a burn rate enhancer in a rocket propellant. (Probably not so great as a fuel, too much carbon and not enough hydrogen.)
There have been many attempts to take advantage of the energy of a triple bond by using acetylene as the fuel component. Unfortunately it’s so darned unstable that the attempts generally have ended in disaster. Maybe graphyne will be less unstable than acetylene. I won’t place any bets either way, though.
Would this be used as a sort of molecular sieve?