Over the years we’ve featured quite a few radiatioactivity detectors, which usually include a Geiger-Muller tube, or perhaps a large-area photodiode. But in the event of radiation exposure from a nuclear attack, how does the man in the street gauge the exposure without owning a dedicated instrument? This was a question of note at the height of the Cold War, and it’s one that [Dr. Marshall Brucer] answered in a 1962 paper entitled “When Do You Leave A Fallout Shelter“. The full paper is behind a paywall but the part we’re interested in is on the freely available first page.
Dr. Brucer‘s detector is simplicity itself, and it relies on the erosion of a static electric charge by radiation. Should you rub a plastic comb in your hair it will accumulate enough charge to pick up a small piece of paper, and under normal background radiation the charge will ebb away such that it will drop the piece of paper after about 15 seconds. His calculation is that once the field reaches around 10 roentgens per hour it will be enough to erase the charge and drop the paper immediately. There’s a comtemporary newspaper report (Page 7, just to the left of the large advertisment) which tells the reader that since the exposure limit is 100 roentgens (one sievert), this test failing indicates that they have nine hours to create a better shelter. For obvious reasons we can’t test this at the Hackaday bench, but those of us who remember the days when such topics were a real concern will be searching for a handy comb anyway.
Thanks [Victor Matthew] for the tip.















Fine as long as you have hair, any suggestions for those of us with bald heads (and wishing to avoid the ignominy of combing our pubes), or for those whose hair has already fallen out due to the radiation?
Rub the comb on a wool sweater or a silk scarf.
Glass on silk. Rubber on fur or wool.
giggity
Pubes won’t work for the same reason head hair won’t work in high humidity climate. Rubbing the rod, ebonite preffered, in necessery I’m afraid.
Make an electrometer with a couple strips of aluminum foil, a piece of solid wire and a class jar or flask and a cork. I used to have my whole HS physics class make them. It takes about 15 minutes to do a good one then the rest of the period is experimenting with them. They were very surprised at making an actual scientific instrument. Maybe the Googler has something …. similar to this oddly complicated one. https://www.youtube.com/watch?v=GA9Kli0XPbw
Pop some fresh silica gell (or re-dried in oven) into the jar to remove moisture from air which will also leak away charge)
I mean, come on.
OBVIOUSLY rub the comb through your massive neck-beard.
The jokes write themselves.
If your hair suddenly starts falling out, I don’t think you need to fiddle around with the comb geiger counter hack anymore. Radiation has been reliably detected.
Better idea….
DONT SPREAD NUCLEAR CONTAMINATION AROUND!!!
“Yeah, but failing that….”
NO! Just dont fucking do it in the first place!
Humans, pfft……and they have the nerve to call themselves ‘great’ apes……
Just comb your back.
Be right back… chasing the cat around the fall-out shelter with a comb…
LoL
I was going to use that child instead
I hadn’t heard this idea before, interesting. I was going to mention humidity has a big effect on this. He does mention humidity near the end of that first page that is displayed.
We all need to create a baseline, run a test in our fall-out shelter now, how many seconds does my comb hold 1/4 of a facial tissue, for comparison to when the bombs have fallen.
Humidity and the fact that your hair will be sweaty and/or greasy after a few hours and ruin the experiment.
“those of us who remember the days when such topics were a real concern”
After Russia’s comments last week, they are a real concern again. Hopefully nothing comes of it but it’s definitely back on the table.
As we have known since the 90s would happen if NATO pushed forward into that country and/or Georgia. Countless national security experts have commented on it (including the current admin’s head of the CIA).
Nato doesn’t push anywhere. Nations aware of how russia behaves join it.
Stop the propaganda already.
NATO isn’t an innocent bystander in the process. They’ve long refused membership applications, but now actively engage. So yes, by now taking in members in an eastward moving direction, they are pushing towards Russia’s borders.
NATO is not pushing forward. Russia can recruit countries to join an alliance. Russia just chooses to do it by force which is why countries VOLUNTARILY join NATO.
” but it’s definitely back on the table.”
I thought during a nuclear attack, we are supposed to go under the table.
B^)
I wonder if you could build a simple radiation detector with static elecricity generator and a hall sensor to measure the drop over time.
What does a Hall sensor sense in your wonder radiation detector?
Well, you could have an attracted plate that swings up to meet the attractor, and then just put a small magnet at the hinge point, whose rotation you detect with the Hall sensor.
Having said that, measuring capacitance might be easier to do.
Even a human hair is too stiff as a flexure for a Kearny-type ‘fallout meter’
It’s highly doubtful a hinge or flexure capable of supporting a magnet will be budged by the electrostatic forces involved. You’d be better off omitting the magnet and just observing it optically.
So the question remains: How would a Hall sensor be used here?
Obviously, you’re just not thinking creatively enough.
Following your suggestion of using optical sensing, you have a camera watching for the falling item. Then you put your camera on a servo mount to keep the falling item centered in its view. (You can use OpenCV to find the object in the image.) Then you put a magnet on the camera mount, and use a hall sensor to detect its position.
You see, there is no situation so simple that you can’t over-complicate it by adding extra parts. There’s an engineer by the name of Goldberg who did a lot of great work in this field.
Sure, someone else might approach this problem with an LED and a light sensor, but what fun is that?
“Sure, someone else might approach this problem with an LED and a light sensor, but what fun is that?”
Using an LED as a light sensor too?
A very simple circuit with a JFET (with the gate disconnected and used as an antenna), a LED and a 9 V battery can serve as a simple electroscope. In post-nuclear times, with a dwindling supply of new batteries, an electroscope can also be constructed with a strip of aluminum foil. The self-discharge time of either can be a good indicator of the level of ionizing radiation.
There’s also the use of an LDR as a detector.
https://www.mdpi.com/1424-8220/20/6/1568/htm
For Xrays… might not work for ionizing rad… low energy fall out isotopes, might not trick the neato chip into making those electron pairs
And for very high dose rates. From the paper it can be seen that LDRs are inexpensive and reliable sensors for dose rates above 50 cGy/min. Roughly, the equivalent of 3000 Roentgen/hour. A dose of 1000 Roentgen, for example, is considered fatal. If you can measure a variation in the dark current of an LDR, you’ll be dead in no time.
I have wired a fet transistor to a meter leaving the base open as an antenna. It makes an excellent static electricity detector – they used to call it an electrometer.
Re: humidity – I wonder if you might be able to build this in a glass jar that wouldn’t impede radiation, use a regulated HV supply and calibrate it?
Probably The Simplest Radiation Detector You Already Own is your cell phone camera.
At the dose rates this ersatz radiation detector is said to detect, your cell phone camera images will be full of speckles.
10 roentgens per hour is 100 mSv/hr which is still pretty spicy. I’m tempted to test it though.
Well, you don’t have to be in the same place, so you can be safe.
And you don’t have to worry much about the phone: I zapped a 1 GB SD card with 10 kR (~100 Sv) (i.e, enough to promptly kill a human). It survived with not a single bitflip. Not quite so extreme doses to digital cameras (SLR & P&S) and a HP41C calculator were similarly uneventful. I should try again with a modern phone.
Spiderwort can detect radiation. I can’t remember the details, but it was news in 1979.
Part of the flower changes color after a few days of exposure to a meaningful amount of radiation.
In 1979 the news also said to hide from a nuclear bomb und a desk. You’ll forgive me if I want a second opinion. 🤣
In 1979, it was mostly about fallout from a nuclear plant.
By 1982, it was about nuclear weapons.
They don’t make desks like they use to.
That was to protect you from falling debris like ceiling tiles, light fixtures, etc.
Though, if you’re close enough for your building to be shaken that hard…
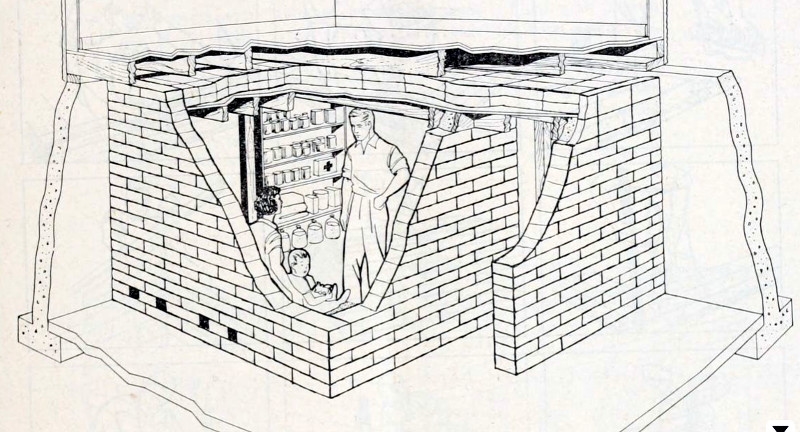
I have old Civil Defense dosimeters that work on exactly this principle. You will also need a dosimeter charger to reset them to zero. Also the main picture is the fallout shelter thats in my basement. The real estate listing said “included wine cellar in the basement”. My house was built in 1959. Heres a link if you want to build one https://www.youtube.com/watch?v=OHmGn-oL2uU
You can also use an electric fly swatter. https://youtu.be/ZBHIp967TD8?t=21
After the fallout alters the genetic make-up of flies, a shotgun might be needed to kill them.
B^)
You can’t generate a static charge with a comb anymore because combs are not made of rubber anymore. This article says “plastic” comb, but Marshall Brucer lived in a time when combs were made of rubber. If you go to Wikipedia you’ll see that says to use a rubber comb. It’s an important distinction.
If you want this to work, rub a rubber balloon on your head, not a plastic comb.
Some combs do it. Depends on the plastic, but that isn’t usually obvious from the comb. I know I demoed it for some kids in the fam a few months back with a purple comb I had, but this black one that’s to hand now isn’t doing much, wonder if the pigment in it is carbon black. Anyhoo, used to be an am-sci site with a handy table of best ones to use with each other, what materials charge each other up best, but having trouble retrieving it. IIRC polystyrenes get a decent charge from hair/wool, but can’t remember the others.
Ah, here we go,
http://soft-matter.seas.harvard.edu/index.php/Triboelectric_series
We see there that nylon combs probably won’t do crap against hair, but vinyl or acetate should be good… basically you want top end of table against bottom end of table, but if it’s conductive it’s likely to bleed off real quick.
This won’t work. If there is so much radiation that the air is conductive you are directly in a fallout zone and nothing will change that. Perhaps a few months of rain that force the particles deep into the soil. Hopefully snow covering it preventing dust being blown up again from wind.
Yeah the only information this will provide you is when to put your head between your legs and kiss your ass goodbye
there is a stark difference between 100s of mSv/h and single to double digits of Sv/h…the former means you could probably leave the fallout zone without any serious ARP, while the latter will probably give you a lethal dose before you can get away.
A few weeks at most will do this as the short-live isotopes decay.
You obviously still need a way of not absorbing the radionuclides while traversing the fallout zone.
“You obviously still need a way of not absorbing the radionuclides while traversing the fallout zone.”
Nuka-Cola, now fortified with potassium iodide!
Or make a lyden jar detector with a glass jar with a lid, some wire and strips of aluminum foil.
This is the same principle that a dosimeter uses. You can build a much better version that takes care of the moisture problem, and is more accurate. As someone who grew up during the Cold War, I had a copy of “Life After Doomsday”, where this design was featured.
https://www.abomb1.org/pdf/kfm_inst.pdf
https://www.youtube.com/watch?v=9eLN0P-SCcU
Here is an version that was updated by Kearny himself.
https://web.archive.org/web/20121030174241/http://www.ornl.gov/~webworks/cppr/y2001/rpt/112538.pdf
I found this old static discharge brush that uses this principal to remove static charge from whatever using polonium.
https://www.reddit.com/r/Radiation/comments/wtzi3j/always_looking_for_elements_for_my_element_table/?utm_source=share&utm_medium=web2x&context=3
Used a similar brush to remove dust from film negatives prior to putting them in the enlarger to make a print..
Do not Google for SciHub and put in the searchbox of this illegal website the DOI number of the closed access paper. It’s completely illegal and I have to warn you about the consequences. I repeat, DO NOT GOOGLE FOR SCIHUB.
Also, DO NOT GOOGLE FOR “GOOGLE”.
I only use Altavista via the Wayback machine. It’s far more reliable.
If you’d like to read a bit of science fiction about surviving nuclear fallout, that contains a bunch of articles on how You Too Can Survive, try the late Dean Ing’s “Pulling Through”
https://www.fantasticfiction.com/i/dean-ing/pulling-through.htm
I wonder if one can do something with a gold leaf electroscope type setup… say you use chocolate bar tinfoil, which is typically quite thin, in place of gold leaf, or possibly metalised mylar… and you have an optical sensor to determine when the leaf drops (apparatus discharged) then one could maybe have an ATTiny85 (or a 555 timer with stable oscillator) topping up the charge at suitable intervals from a voltage multiplier…. or motorise a medicine bottle against a discarded silk tie belt, so then if leaf drops in interval radiation is dangerous level… this sets off the beeper though the digital magic of your ATTiny85 or analog magic of your 2N2222 light detector.
Actually I wish there were some better guides online for how to repurpose the ionisation detector out of old smoke alarms without doing anything stupid with the Americium.
Here you go. A DIY radiation fallout meter.
https://info.ornl.gov/sites/publications/Files/Pub57069.pdf
10 Roentgens per hour?!?! That is extremely hot and I doubt ones long term outlook would be that good. I have two mil surplus AN/PDR-27 units and they top out at 500 milliroentgens. Even that is a level I would not want to be around too long.
Hey, who doesn’t want to go through life as a nightlight.
Mind that 100 mSv is the lower limit of statistically significant cancer risk. Anything below that is extrapolation, and the workplace radiation safety limits for lifetime exposure were arbitrarily placed at 20 mSv simply to err on the side of caution. You need 1,000 mSv total exposure to have 5% chance of eventually developing cancer.
10 Röentgens per hour is 10 mSv per hour. All the panic about how you’d be walking dead within hours is based on a misunderstanding of the radiation safety standards. Even after a few days, you’d still have a 95% chance of nothing special happening to you.
To put things into perspective, 1 Sv is equal to 1 Gray of absorbed dose, which is 1 Joule of energy per kilogram of body mass. 10 mSv/h is about 0.8 Watts for the average person. This is roughly the same full body dose rate of ionizing radiation as you would get by laying flat on the ground naked on a sunny day (UVB light).
Sorry, forgot that Röentgens to mSv is 10x not 1x, so you’d get 100 mSv per hour. Still, you have hours to leave without significant harm and days before you really start to get sick.
Dammit, my brain isn’t working. If it’s 80 Joules per hour, then it’s 22 milliWatts, not 0.8W.
Meanwhile, the sun is still putting out about 1.5 Watts of UVB per square meter on the ground, so 100 mSv per hour is in the same ballpark as getting a sun tan. Slightly less.
No, 10 mSv/h is 0.22 Watts and 100 mSv/h is 2.2 Watts for an 80 kg person.
Now it is correct.
So much for the ease of SI units when different quantities are still arbitrarily 10,100 or 1,000 times smaller or larger, and there’s a factor of 3.6 tucked in there somewhere.
Dude, how much is that in ergs per inch-pound?
I don’t know, but then again, I’m still not sure that I didn’t get it a factor of ten wrong anyhow.
The 0.8W figure should also be 0.022 Watts and 100 mSv/h would be 0.22 Watts, but the auto-moderation ate the comment.
Based on the image it would have been hilarious to say your kids can be the radiation guinea pigs.
Don’t try a cockroach to sense ionizing radiation.Their little exoskeleton is an efficient shield. At Macquarie uni they placed cockroaches in a pit then gave em heaps. The critters lasted over a month.
The electroscope radiation detection method was actually the basis for cold war era personal dosimeters stocked into civil defense shelters . The device is a small yello tube about 5 inches in length and had a Pen like pocket clip. the device was charged from a charger that worked on 1 d cell battery. you looked in one end of the unit and pointed it at a bright light to read a scale at the far end with a needle indicating the accumulated dose you were exposed to, the unit required no battery its self after it was charged the needle was a mica whisker that was hinged at the top it was in fact a calibrated electroscope. the big problem i see is if 10 units (cant relate to old measures) was instant discharge, how do you know the rate is just at 10 ? how would you tell 10 units / hr from 20,30,50 units an hour??