There’s an old magic trick known as the miser’s dream, where the magician appears to pull coins from thin air. Australian scientists say they can now generate electricity out of thin air with the help of some enzymes. The enzyme reacts to hydrogen in the atmosphere to generate a current.
They learned the trick from bacteria which are known to use hydrogen for fuel in inhospitable environments like Antarctica or in volcanic craters. Scientists knew hydrogen was involved but didn’t know how it worked until now.
The enzyme is very efficient and can even work on trace amounts of hydrogen. The enzyme can survive freezing and temperature up to 80 °C (176 °F). The paper seems more intent on the physical mechanisms involved, but you can tell the current generated is minuscule. We don’t expect to see air-powered cell phones anytime soon. Then again, you have to start somewhere, and who knows where this could lead?
Microbial fuel cells aren’t new, of course. If you just want lights, you can skip the electricity altogether.













It’s a matter of scale. We may not be able to scale this up now, but who knows what the future holds?
The possibilities….
Running a remote monitoring station off of it.
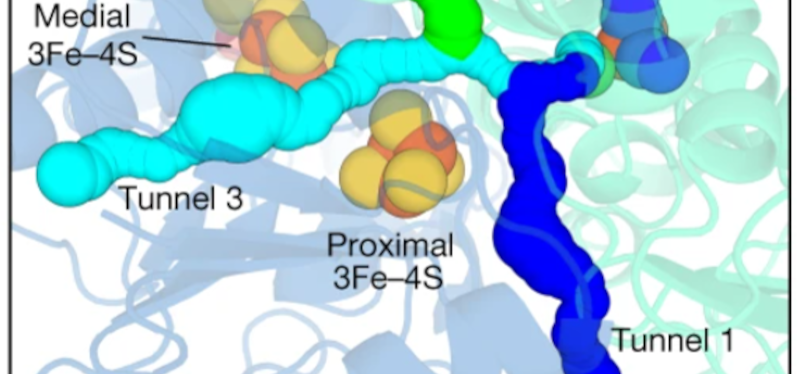
The enzyme can easily be loaded on a support to convert H2 to electricity. Should result in higher efficiency electricity at low temperatures.
Where Carnot might reign for heat engines, Michaelis and Menten would have a word or two to say here.
It’s an odd sort of atmosphere that would have enough free hydrogen in it to make this work.
This is a classic case of overinterpretation by the press in the interests of sensationalism.
You aren’t going to be charging your car or even your cell phone any time soon. There just isn’t enough hydrogen in the atmosphere (about 0.00005%).
The research, which was masterfully done, answered two questions, neither of which will keep you or I awake at night with excitement.
Question 1: Why is there so little hydrogen in the atmosphere?
Question 2: Why do certain bacteria survive and even thrive in the apparent absence of an energy source?
The answer is literally blowing in the wind. The bacteria have evolved a way to capture the meager diet of hydrogen and oxidize it, trapping the released energy and using it to live. In this way, these critters have kept the hydrogen in the air to a vanishingly small amount.
This excites me, but it’s not nearly as thrilling as a car that literally runs on air or a cell phone that will never triple beep.
No, the answer to question 1 is that hydrogen floats away into space. The noble gas helium is rare in our atmosphere for the same reason.
As a general rule, of all of the processes impacted upon by thermodynamics the higher the temperature the more efficient they can be. Biological processes are not particularly efficient and never will be for that reason, they evolved to meet a whole lot of other criteria as well as efficiency, such as being part of a mechanism that can repair and replicate itself. So unless you can devise an enzyme that operates at 800 Celcius…
As Wolfgang Pauli said, that’s such a bad interpretation of “efficiency” it’s not even wrong.
The efficiency of a biological process is not intrinsically related to temperature. Its rate, and even its balance might be, but not it’s ‘efficiency’, however you might define that. More specifically, it’s NOT thermodynamically a heat engine. It’s a chemical process.
Even a heat engine (which I think is where you got the notion) is not “more efficient” at high temperature. In the Carnot sense, a heat engine efficiency is defined by (1-) the RATIO of absolute temperatures it operates at, not its absolute temperature.
If one heat engine operates at an input temperature of 800 C, and rejects heat at 700C, it operates at LESS efficiency of an identical one that operates at 400C and rejects at 300C. Same temperature difference, but the engine that runs cooler is 50% more efficient than the hot one.
And the chemical factory that is my liver operates at an input temperature of 37C, and an output temperature of 37C. What’s it’s ‘efficiency’?
Ignoring your irrelevant straw man about thermal gradients that is just an insult to everyone’s intelligence. Tell your theory to the guys who design nuclear reactors, or more specifically the engines used to convert the heat from them to electrical power. There is a pretty clear relationship between temperature and efficiency, which gets close to 50%.
As for your liver, have you actually checked that claim? It does work, it produces heat as waste.
Again: chemical processes are not heat engines. Trying to apply thermodynamic rules related to heat engines, like Carnot’s Principle, is not even wrong: it simply doesn’t apply.
The statement that a heat engine operating between 400 C and 300 C is more efficient than one operating between 800 C and 700 C is correct. It’s not an insult to anyone’s intelligence. It *is* a challenge to ignorance.
There is no nuclear power plant that achieves anywhere close to 50% efficiency. Many combined-cycle gas turbine can get that though, producing half as much waste heat per unit of electricity than nukes.
And well spotted: a liver does produce heat as a byproduct of its biochemical activity. It raises the temperature of the blood flowing through it about a half degree. If it were a heat engine it would be an overunity machine, but clearly that doesn’t apply.
Igor Pioro – Handbook of Generation IV Nuclear Reactors
“It will take at least two or three decades before the deployment of commercial Gen IV systems.”
Assertion stands.
See the chapter: Hydrogen production pathways for Generation-IV reactors.
You may be thinking about the Arrhenius Equation, which is an expression of chemical reaction rate as a function of temperature, but not at all of efficiency.
Enzymes have optimal temperatures for operation, and the Arrhenius equation does have some effect on the rate of diffusion of substrates through the solution, but the enzymes themselves have ideal temperatures for operation that are entirely dependent on their stability, which is largely determined by things like van der waal forces that hold them together. Like, ribulose decarboxylase/oxygenase is part of an enzyme complex, and by itself has a conversion rate that rises with temperature until it denatures, but the other enzymes in the complex act to reduce its conversion rate at temperatures over 35C, and they’re necessary for overall enzymatic productivity, so saying “enzymes have higher efficiency at higher temperature” ignores most enzymatic systems, that are regulated and involve substrate transfer between enzyme groups, for which temperature is one of many inputs to the overall system productivity and definitely not an input that shows a simple increase in throughput as it rises.
Huge vats around the infrastructure for hydrogen powered vehicles, to recover the estimated 50% transmission losses?
Imagine creating hydrogen by electrolysis and replenishing the electricity by burning that same hydrogen?
Environment prep and waste products?