With demand for lithium in the world market projected to increase by 2040 to as much as eight times the demand in 2022, finding new deposits of this metal has become a priority. Currently most of the world’s lithium comes from Australia, Chile, China and Argentina, with potential new mining sites under investigation. One of these sites is the McDermitt caldera in the US, a likely remnant of the Yellowstone hotspot and resulting volcanic activity. According to a recent study (Chemistry World article) by Thomas R. Benson and colleagues in Science Advances, this site may not only contain between 20 to 40 million tons of lithium in the form of the mineral clay illite, but was also formed using a rather unique process.
This particular group of mineral clays can contain a number of other chemicals, which in this particular case is lithium due to the unique way in which the about 40 meter thick layer of sediment was formed. Although lithium is a very common metal, its high reactivity means that it is never found in its elementary form, but instead bound to other elements. Lithium is thinly distributed within the Earth’s crust and oceans. Incidentally, the Earth’s oceans contain by far the largest amount of lithium, at approximately 230 billion tons.
So how much lithium could be extracted from this new area, and how does this compare to the increasing demand?
Chasing The Buck
Probably is mostly the concentration of the lithium that determines whether extraction is economical. In ocean water, the concentration is approximately 0.14 to 0.25 ppm, whereas that in one of the currently largest known reserves in Chile is 6% after evaporation of most of the moisture in the pumped-up salty water. In comparison, the approximate concentration of lithium in the illite mineral clay samples analyzed by Benson et al. was over 10,000 ppm. This is significantly more than is contained in the other common mineral clay – smectite – also found in the McDermitt caldera region at 3,400 to 6,800 ppm.
The complicated distribution of the lithium deposits is due to the volcanic origin of the region, which in a sense acted to concentrate lithium not unlike how lithium is recovered from salty water by concentrating it into brine.
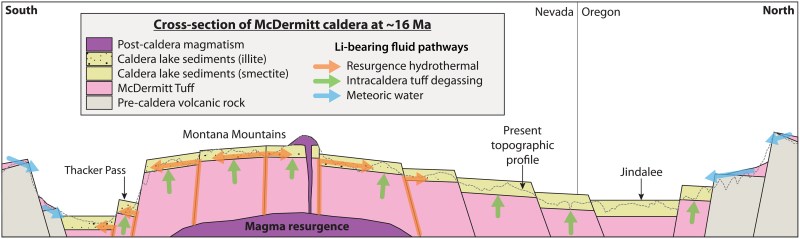
Just knowing the concentration of lithium in a few select samples isn’t enough, of course to determine how much lithium might be extracted. This is where the geological study of the region comes into play, which was described in a 2020 study published in Minerals, carried out by Stephen B. Castor and Christopher D. Henry. This study builds upon previous geological studies that examined the formation and current organization of the region’s layers.
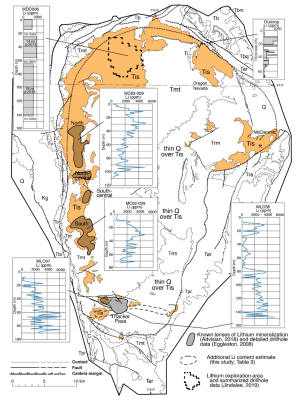
Previously this part of the caldera – called Thacker Pass – has been investigated in the most detail, with its smectite deposits already being an attractive target for mining. The newly studied illite mineral clay would seem to be an even more concentrated result of the same processes that would have formed the smectite sediment. In the article by Benson et al., it is proposed that this process involved successive volcanic resurgences, along with the presence of a lake in the caldera in between. The caldera and the lake would have gathered and concentrated a large amount of minerals and other elements using a hydrothermal process that ultimately resulted in the meters-thick layers of mineral clay sediment found in the region today.
Here opinions between Benson and Henry differ, with the latter seeing an issue as the geological evidence indicates that the lake persisted long beyond the era of volcanic activity. Regardless, the presence of these sediments are an empirical fact, and the Lithium Americas Corporation where Thomas R. Benson is employed as a geologist intends to begin mining these illite deposits by 2026. At the projected concentrations of the millions of tons of lithium contained in the clays it should not only be economically viable to extract, but also last for quite a few years.
In 2023, the global demand for lithium is projected to be about 685,000 tons, in the common lithium carbonate equivalent, to rise to more than 2 million tons by 2030. Despite the association of lithium with renewable energy, the mining and processing of lithium from both brine and other sources is highly controversial due to the environmental impact. The cause of this is mostly the high water usage with brine extraction, as detailed by Maria L. Vera and colleagues in a 2023 review article in Nature Reviews Earth & Environment.
Environmental Issues
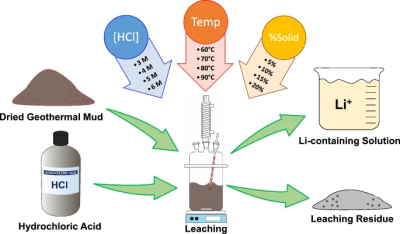
When processing lithium-containing clays, the use of sulfuric and other acids is very common, in a process called acid leaching. The consequence of this is that in order to safely mine lithium, environmental considerations have to be taken seriously, as this tends to produce large amounts of hazardous waste. It is perhaps little surprise that many environmental groups have lodged protests against lithium mines in Chile and elsewhere, as lithium extraction from brine, hard rock and mineral clays all come with a range of issues.
When lithium extraction begins at the McDermitt caldera, dealing with these issues will be an important aspect, along with the energy required for the extraction process. This incidentally is an issue shared with the recycling of lithium-containing material, such as battery cathodes, with Yi-Chin Tang et al. (2023) studying the efficiency of acid leaching with three different types of acids. Here sulfuric acid was the most efficient, recovering 71% of the lithium after 15 minutes at 60°C, yet leaving acid that has to be safely disposed of, a loss of lithium material with each processing and recycling step, and the need for energy input to get the appropriate reactivity temperature.
Terra Incognita
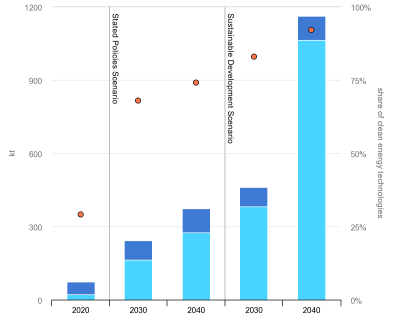
According to the International Energy Agency, the demand for lithium is expected to increase from 74 kilotons in 2020 to an astronomical 1,163 kilotons in its ambitious ‘sustainable development’ scenario, or a 15.7 times increase in twenty years. Considering the lead time before a new lithium mine can be prepared and started, this would seem to be more than just somewhat ambitious.
If such a spike in demand were to become reality, it’s not hard to see how even a few dozen megatons of lithium deposits could be used up in a matter of decades, even with recycling in place. A lot of total demand for newly extracted lithium will depend on how much recycling will truly be possible, and what the losses will be in the process.
Another major unknown is whether or not the extraction of lithium from seawater becomes possible in a way that makes it economical. This is incidentally where research in the extraction of uranium from seawater could conceivably also provide some inspiration. Regardless, how the demand and supply sides of the global lithium market will develop over the coming decades will likely remain the territory of speculative investors, even if this potential new source of lithium in the US could help to significantly ease the supply within the immediate future.

















Talking of resources.
I heard that one side of the moon has a lot of iron and the other side a lot of aluminium.
Now with the moon being so dusty from ground up material does that mean that if we accidentally land a lander on the dividing line we ignite the moon as one big ball of thermite you think? That would be surprising and raise some heads I imagine.
I wonder if we could feel the heat.
no
Thermite is made of powdered aluminum and ferrous (iron) OXIDE. That last part is important, as it is the reason it does not require external oxygen to burn. Aluminum and iron together at high heat would just result in them melting together, rather than the aluminum and oxide portion burning up and leaving elemental iron behind.
I am not a mineralogist, but with the absence of an oxygen-containing atmosphere, I don’t think there are a lot of oxides on the moon. I could be wrong, though.
The thermite mix would also need to be at sufficient concentration in the dust to ignite and burn properly, not to mention to spread the ignition. I’m guessing the mineral composition contains far too many other elements blended in sufficient concentrations for it to not ignite, even if it were mostly ferrous oxide instead of plain iron.
I’ve made a variety of thermite and thermate mixes and blended them into stuff like clay and later, various better binders. Depending on the concentration, you can either get significantly reduced temperature output or no ignition at all. I got the clay idea from instructables, but that was definitely the worst mix.
Also, remember, the moon is essentially our asteroid catcher – it has seen A LOT of big impacts over the ~4.5 billion years it’s been around. Those impacts almost certainly released more energy than any lander could, and yet, or never turned into a ball of fire. For that to happen, it would basically have to be a giant ball of well-mixed, finely powdered aluminum and some form of ferrous oxide. Gravity alone would end up separating the two quite a bit over millions of years, simply because they have different mass per volume.
+1 Good response! No, *great* response!
Actually, nearly all the iron, as well as all the aluminum on the moon is in oxide form. Said oxides probably forming while the solar system was still condensing rather than by geo/senological processes. The reason the moon is not made of thermite is that ALL the metal is in the form of stable metallic oxide minerals.
For your thermite reaction you need iron oxide and metallic aluminium. In reality I expect both metals to be in oxide form on the moon. So no reaction.
It may be utter nonsense but I’d watch the Michael Bay disaster movie of the frickin’ moon exploding.
Most of the dust is a mix of silicate and other mineral, not iron or aluminum. And beside aluminum doesn’t exist in pure form, it only exists in other alloy like bauxite.
Whatever happened to getting it from the Salton Sea? That was supposed to be a large source AND it was supposed to be environmentally friendly.
Once you suck the water in it becomes ‘toxic waste’ and is your problem, so nobody will.
The first extraction facility is expected to come online in 2024. https://www.cnbc.com/2022/05/04/the-salton-sea-could-produce-the-worlds-greenest-lithium.html
A growing and largely untapped resource of highly concentrated lithium is used batteries.
First we have to ban throwaway vapes with lithium batteries. Go into any convenience store and look up at a wall of them.
Why? They are a great source of free batteries for small projects. Just look in any litter bin, or pavement.
‘Exploding demand’ in an article about Lithium for batteries?
I can’t make my mind up if it was intentional or accidental genius
The safety concerns and cost are worse for this new discovery as the mining rights owner is proposing open pit mining of massive areas and “borrowing” 1B gallons from the great salt lake exposing toxic sediment to be blown straight at SLC. Not to mentioon the water treatment required to restore it to original state
No. Thacker Pass is hundreds of miles from the Great Salt Lake.
I have heard we here in ‘Murica have enough lithium to meet future demands for a long while. Lotsa mineral rich saline deserts. What we don’t have is much of the refining processes sufficient to process what we could mine.
Even under favorable circumstances it would take a decade of permits and envirinmental studies. Not to mention the same people who want electric cars chaining themselves to bulldozers or just lighting them on fire.
Is this the same lithium deposit that NASA vetoed the mining rights for because it uses the terrain as a calibration target for satellites?
I keep reading in the local news they found a huge lithium deposit in western maine but local laws will basically make it impossible to mine.
I may be an enginerd but mixing ppm and percent in the same sentence still does my head in.