Science and engineering usually create consistent results. Generally, when you figure out how to make something, you can repeat that at will to make more of something. But what if, one day, you ran the same process, and got different results? You double-checked, and triple-checked, and you kept ending up with a different end product instead?
Perhaps it wasn’t the process that changed, but the environment? Or physics itself? Enter the scary world of disappearing polymorphs.
Point of No Return
Imagine you’re working at a laboratory that creates pharmaceuticals. You figure out the reactants and the chemistry involved to make a new drug. You design a production line, and your new factory starts churning out the drug in mass quantities. This goes well for years, until suddenly, the drugs stop working.
You run an analysis, and the drugs coming out of the factory aren’t what you designed at all. They’re a weird new form of the same chemical with a different crystal structure, and they’re no longer working the same way. What happened?
You’ve probably come across the case of a disappearing polymorph. This is where the original version of a chemical’s crystal structure becomes impractical or impossible to produce. Instead, you tend to end up with a new version instead, typically a more stable, lower-energy version. The mere presence of this newer, more stable version tends to convert the original version into the new form quite easily. Since the new form is more stable, it tends to become difficult to convert the product back to the original form, or functionally, to produce it at all.
Paroxetine Problems
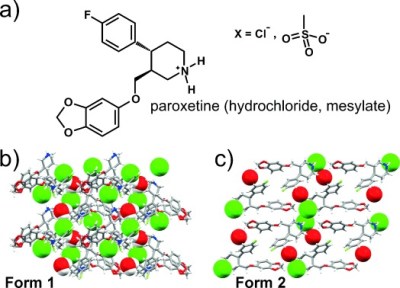
A great case study exists in paroxetine hydrochloride, an SSRI medication. The initial form of the drug was developed in the 1970s, and was known as paroxetine anhydrate. It was produced as a hygroscopic, chalky powder. However, in 1984, a new version spontaneously popped up at sites in the UK that were scaling up production. The new ‘hemihydrate’ crystal form was more stable. Drug in the anhydrate form would spontaneously convert into hemihydrate whenever the two came into contact in the presence of water or mere humidity.
The issue caused legal problems down the line. Years later, other drug manufacturers wished to produce paroxetine, too. The patent on paroxetine anhydrate had ran out, so generic manufacturer Apotex moved to begin production. The issue was that the company found it could not produce the original form. Instead, its product inevitably came out as paroxetine hemihydrate. It’s believed that the Earth’s atmosphere had functionally become populated by trace amounts of paroxetine hemihydrate, to the point where any paroxetine anhydrate would immediately be transformed into the new structure.
By this time, GSK was the company that held a still-active patent on paroxetine hemihydrate. It sued Apotex, arguing that its generic pills contained paroxetine hemihydrate that had been created through the atmospheric seeding process. The courts accepted GSK’s submission on this point, but ruled in favor of Apotex’s right to continue producing its generic pills. It was noted Apotex could not be held responsible for the issue of uncontrolled crystal seeding.
Later research saw two separate companies independently create another polymorph. Both Synthon and SmithKline Beecham sought patents for the production of polymorphs known as paroxetine mesylate. However, a similar problem cropped up shortly after. Any attempt to create the Synthon polymorph would end up creating the Beecham structure instead. This lead to much confusion over whether Synthon’s version was a new case of a disappearing polymorph, or whether the company had made errors in its patent work. Ultimately, no satisfying consensus was reached as to the truth of the matter.
Treatment Failure
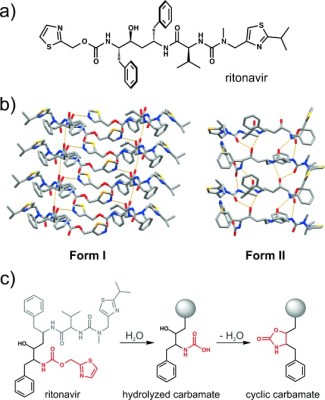
Sadly, disappearing polymorphs can create more than legal woes. Ritonavir was released for public use in 1996, a crucial antiretroviral drug used in the fight against HIV. In its original crystal form, known as ‘Form I”, it was quite soluble and medically useful for treating the condition. However, in 1998, “Form II” was discovered. This was a more stable polymorph that existed at a lower energy level. The problem was that this crystal form was much less soluble. This made the drug less bioavailable, ruining its effectiveness at treating the disease.
The existence of Form II threatened the production of the useful form of the drug. Any laboratory that saw the introduction of Form II was unable to produce Form I afterwards. It was speculated by researchers that individuals that had worked in such labs could carry traces of the new form, and potentially poison facilities that were still producing Form I. In the space of a few weeks, everywhere that could once produce Form I was rapidly turning out only Form II instead.
Due to problems with production and the lack of efficacy of Form II ritonavir, the drug was pulled from the market. This lead to thousands of patients going without medication for their condition, and losses of over $250 million for the manufacturer, Abbott. The company held press conferences that highlighted the gravity of the issue.
“This is why all of us at Abbott have been working extremely hard throughout the summer [of 1998], often around the clock, and sometimes never going home at night. We have been here seven days a week and we will continue to do so. We have cancelled vacations and asked our families for their understanding and support. This is not an issue that we take lightly.”
Eventually, the problem was overcome. Researchers found a way to produce Form I under highly controlled conditions. The product had to be sold in a special refrigerated gel cap, compared to its original delivery form of a non-refrigerated capsule. Later developments included a combination of lopinavir and ritonavir that did not require lower temperatures to remain stable, and a new form of melt-extruded Form I ritonavir tablet that hit the market in 2010.
What Can Be Done
Scientists are some what at the mercy of nature when it comes to disappearing polymorphs. New polymorphs can pop up without warning, while tiny seed crystals can quickly contaminate entire labs, factories, and indeed, the world. There’s little defence. The only solution is doing hard chemistry—either to find ways to make original polymorphs survive the new world, or to find other new polymorphs that are still useful and still producible.
Ultimately, though, new polymorphs can be a pharmaceutical engineer’s nightmare. They can ruin a drug and ruin a factory overnight. Stories of ritonavir and other drugs will remain cautionary tales for this very reason.















I never knew. Brilliant and shocking. This is why HAD is must reading!!
Derek Lowe’s IN THE PIPELINE even more ;)
Ice-9, for reals
“By this time, GSK was the company that held a still-active patent on paroxetine hemihydrate. It sued Apotex, arguing that its generic pills contained paroxetine hemihydrate that had been created through the atmospheric seeding process. ”
Amusing they blamed them like it’s some kind of conspiracy.
Not dissimilar from the big Ag companies that sue small farmers when the wind blows pollen from neighboring GMO fields, because the small farmer’s crop now contain the patented GMO genetics through no fault of their own
If those small time farmers can find a decent lawyer who works pro bono, they could counter sue for entrapment by “polluting” their farm with company’s patented GMO material then suing them in the first place. Or something like it.
Only lawyers working pro bono could survive potentially costly ligation. Farmers themselves would run out of money and be unable to defend themselves and lose their farm to sloppy GMO companies.
In the most commonly cited example:
The farmer wasn’t denying that he sprayed the second years crop with Roundup equivalent.
Thereby selecting resistant examples for 3rd and later years crop and continuing to use (generic, the real problem) Roundup.
He just said: ‘Neener!’
Showed his ass.
He should have started with ‘prove any of it’.
Here is your trespass notice. Notice the broad language.
Here is another. Narrower. Just you, your employees and contractors.
Chem companies just revised the plant genetics and Roundup chemistry when patients expired.
Bet you money there are private stock of old roundup resistant seeds in farmers hands.
Being quietly planted and harvested, year after year.
Animal feed. Human food too closely inspected.
Exactly what it made me think of.
https://en.wikipedia.org/wiki/Ice-nine
Great reference; thanks.
Ditto!
I was amazed when I got to the end of this article without a single mention of ice-9.
Bokonists usually keep quiet for fear of “The Hook”…No footplay!
FYI, Ice-0 has been recently discovered.
https://scitechdaily.com/ice-0-scientists-discover-unusual-new-form-of-ice/
Reminds me of the chirality problem with thalidomide. It comes in left-handed and right-handed molecules and they didn’t realize that the right-handed configuration caused birth defects.
Or, more importantly, that the two enantiomers convert back and forth in the body. Administer pure R-thalidomide and you still get teratogenicity.
Polymorphs are a hugely interesting and deeply problematic. Raman spectroscopy happens to be one of the most widely used tools to differentiate between polymorphs, and has seen a substantial increase in use because of this application.
Polymorphs also make me think of prions, the protein equivalent. Scary stuff: Ice 9, but not imaginary.
I’ve never had much interest in chemistry, but this was fascinating!
Wow…great article. Never knew about disappearing polymorphs. I wonder if D.P. ‘s can be included as a subset of Chaos Theory. I don’t want to give the benefit of the doubt to big pharma but this is one good reason why drug manufacturing is not a perfect science.
“It’s believed that the Earth’s atmosphere had functionally become populated by trace amounts of paroxetine hemihydrate, to the point where any paroxetine anhydrate would immediately be transformed into the new structure.”
This is a hard one to swallow or breathe….and everyone is worried about CO2….
And all of the estrogen from birth control pills that pass from the body and through sewage treatment intact are messing with aquatic fauna.
Sexy fish.
In our water, greatly diluted.
If Homeopathy was true, all the women would be fertile as all F.
Knocked up from a passing man’s horny thoughts…That and the F-ing.
Also:
MAKING THE FROGS GAY!
Left as exercise:
Diluted Viagra.
Oh yeah. Pharmacology is literally everywhere, can’t put the cork back in that bottle for a good long while..
It’s kinda similar to pre WW2 iron being more valuable because it’s missing the background radiation from the age of the atomic bomb (tests). https://en.wikipedia.org/wiki/Low-background_steel?useskin=vector
Sounds a lot like dark matter, a lot of “we don’t know, so…” When two polymorphs love each other very much… Do we have eyes on Prof. Phlogiston? Bueller? Bueller? What’s the cleanup schedule? You’re not going to get quality pharms out of a McDonald’s slushie machine.
But those poor people! They worked so hard, so very hard. Too much work is aggravated their condition. They should take it easy.
It’s a disturbing thought that since 1984 the atmosphere had enough SSRI medication in it that it made production of paroxetine impossible. Really gets the noggin’ joggin’.
I made a comment on slashdot years ago about programming becoming more difficult due to the increased complexity of computers. A programmer replied and agreed, saying we might be heading for a black swan event.
This seems like a better candidate for a black swan. for instance, imagine if something similar happened with diabetic insulin, which caused the natural insulin in human bodies to change to a type incompatible with life. then all the healthy persons would die, and only the diabetics would survive.
Telltale: The Walking Living.
If you want some extra existential dread, it’s also possible that the universe itself is a false vacuum and could undergo spontaneous decay to a new lower-energy state. Suddenly, all the rules of physics could change arbitrarily in ways completely incompatible with any current life.
(of course, the collapse would happen at the speed of light, so it’s probably unlikely such an event would reach us… but we also wouldn’t have any warning if it was about to.)
In the 2005 movie Serenity, on the planet Miranda the Pax or G-23 Paxilon Hydrochloride was added to the air processors to calm the population and reduce aggression but it had 2 effects. Most of the people stopped doing everything….eating, breeding, working and starved to death. In the rest it magnified their aggressor response beyond madness creating the violent Reavers.
Maybe we are slowly drugging ourselves to death.
Side effects: Reduced aggression…drowsiness…digestive upset…heart palpitations…light sensitivity…flushed skin…elephanttitus…reaving.
Oh, I need to find the corollary for doing something successfully several times and then the subsequent attempt doesn’t go so well.
I’ve made this recipe for (chocolate chip cookies, brownies, carrot cake, etc.) so many times in the past, but now I can’t seem to get it right. Curse you disappearing polymorphs!
If I remember correctly, production of synthetic quartz crystals ran into an issue with an undesired polymorph taking over completely, though the issue was eventually resolved. Also consider prions, in which one misshaped protein can cause all other molecules to assume that pathogenic form, as in Creutzfeldt-Jakob disease, Kuru, scrapie and similar transmissible spongiform encephalopathies.
This is so many adjectives ranging from fascinating to Horrifying And if it did make it on a news, I don’t think it was more than a 10 second segment.Or a ticker tape item.
I still remember the 90s.
When they show this one segment, about 1/3 of the.
Polar ice shelf had cracked in half or cracked off.
And they went directly into sports. It was. Trying to convince somebody you saw a ghostly shadow go across the room.
Did you see it?Did you see it? Wait come back it was important!
We’re Doomed 😧
But thank you all of you. I realized there are some people paying attention.✌️👍
Makes me think of Cat’s Cradle and Ice Nine but IRL.
Neat! I learned something today. Thank you!!
So it’s the chemical equivalent of your code working fine in testing but then not working in production for no perceivable reason.
I’m not a chemist but I know enough to be dangerous (to myself). This makes no sense to me. If this were a supersaturated solution and the contaminates were seed crystals that that couldn’t be 100% eliminated I’d understand how it was possible to create a supersaturated solution in the past but it can no longer be done.
But polymorphs sound like isomers to my uneducated background. The transition from one isomer to another has an activation energy. You have to rotate a bond or transfer an electron or two from one form to achieve the other. Sometimes a catalyst helps facilitate the transformation. But why does the presence of a lower energy variant of a polymorph act as a catalyst to that form? And from a statistical mechanics point of view, would we expect ALL forms of the polymorph to exist at the same time even if some forms had a low probability of forming. (I’m thinking quantum tunneling here.). And so, even if there’s a one in a trillion chance of forming the “killer” variant that’s pretty much a guaranteed scenario after a couple of months of production, right?
It sounds like the presence of the lower energy polymorph is correlated to the inability to generate the previous version but I don’t understand the casual relationship.
Which is probably why I’m not a chemist. Can anyone help explain this to me?
In the case of the hemi/anhdrate the material was hygroscopic therefore attracting water/ and the hemihyrdrate has water so something like that can often share noncovalant bonds with water if I understand correctly.
As anybody who’s tried to dry/ distill glacial acetic anhydride from an aqueous solution can attest to to its nearly impossible to remove all the moisture.
Rupert Sheldrake has some interesting thoughts on this, he calls it morphic resonance. I’m too tired to write out a helpful summary on it atm, but look up his name + morphic resonance and there will be some talks on it, and a book or two. Smart guy, not saying its 100% correct, but since nobody knows the answer, non standard theories should be discussed imo