While you tend to think of diamonds as ornamental gemstones, diamonds also have many important industrial uses, and many of those diamonds are now synthetic polycrystalline diamonds. How are they made? [JerryRigEverything] takes us behind the scenes at a diamond manufacturing facility, something you don’t get to see every day. Check out the giant presses that exert about a million pounds of pressure in the video below.
The process starts with diamond powder, which is just what it sounds like. Although you can get real diamond powder, most uses today start with synthetic diamonds. The powder has many uses in cosmetics and as an abrasive. But the video will combine it with cobalt and table salt to form diamond shapes.
The salt is a high-temperature electrode. The process requires temperatures of nearly 1400C (2500F) and a lot of pressure. Common talc, some metal electrodes, and a heater tube are also used in the process.
The press can convert a little diamond dust into a diamond in about 10 minutes. However, because the machines are so dangerous, they are each set in their own blast room, which is sealed when the press is in operation.
While this press was — no pun intended — impressive, we’ve seen bigger. Nothing like this will show up in your garage anytime soon, although, as the video shows, you can buy 3D printer nozzles made from the material. As for a press, you might have to just settle for an arbor press.

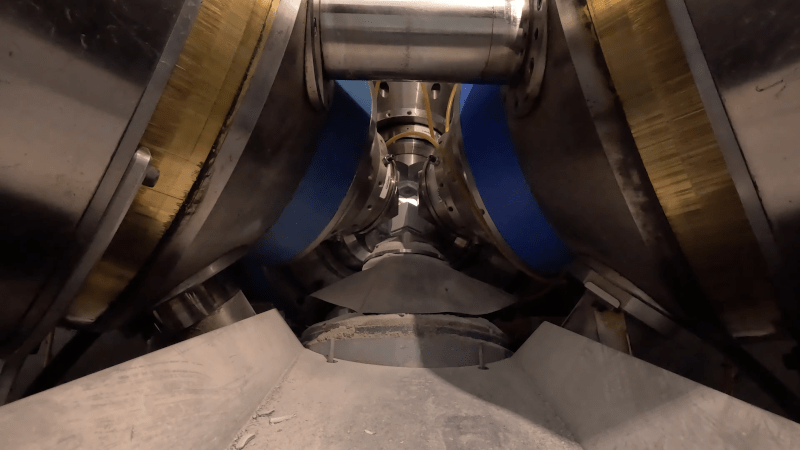














Does anyone know why these machines are built in this way? Best I know, the sintering temperature is so hot that the salt melts and becomes a liquid. So why not just use a cylinder to apply force from one side? I already saw this video a few days ago, (the youtube algorithm), and although interesting enough to watch, it is light on detail.
You can also buy these things from Alibaba, (Search for “diamond press”) but they look a bit different. Instead of the “round bars” connecting the sections, there are heavy “chain links”. The sections are also from cast steel instead of (probably) turned.
its engineering right
so the “3d” press is going to be the pragmatic way to get a larger
“ingot”, and the Chinese are going after faster ,cheaper , high volume
in all senses, but will sacrifice tooling failure to keep costs down
and so syntetic diamond production is growing rapidly, as is the size of the finished diamonds.
while the chinese presses look rough, I am very curios about the metalurgy behind there castings.
the chains on the chinese presses might be for “saftey” and just catch the press head, when it fails, rather than having the expense of
running the press inside a bunker, but I bet that there is no one in the building, when while a press run is happening,
It’s about 725,000 psi. Would that cause considerable damage to my garage?
My guess is that it is pretty tough to contain that kind of pressure if you apply it from only one side. Most things get pretty elastic at such forces. And the end result is probably ruined if the pressure isn’t even across the crystal
Just a bit disappointed that the process starts with diamond dust vice say, a lump of coal.
miracle of commodities…every step can specialize to the material generated by a previous step
I wouldn’t say complexity is a miracle.
I don’t know, I think life is pretty miraculous, and you don’t get much more complex than that. And most key biological processes are based on compounds produced by other seemingly unrelated processes. All built in a cave… from scraps.
Complexity is more about how much information it takes to describe a system. In that sense, things that are borderline chaotic are more complex than ordered systems. No matter if it looks complicated or has many steps, as long as it’s operating in a simple fashion that is easy to describe and keep track of all the moving parts, it’s not complex.
Complexity takes more energy to sustain than ordered systems, which is why complex systems tend to reduce to simpler ordered systems over time – or rather, what remains is the ordered system while everything else stops moving due to a lack of energy.
hello first think reading about that thinking are that repeatable about crazy idea are just 4 gpa press and welder electrolytes
It’s a triaxial press. It applies force in 3 directions. There is no other way to control a hydraulic confining fluid at that pressure level. The melted salt forms the confining force. These have been used for many years for experiments in high pressure, high temperature physical chemistry. The crystal structure of quartz depends upon the conditions in which it was formed as do most minerals.
The formation of a diamond requires duplicating the conditions in which carbon takes that crystal form. It’s been over 42 years since I completed my MS in igneous petrology. This is the standard lab equipment for experimental studies of the phase chemistry of minerals scaled up by the application of a lot of money. In this case they are merely sintering diamond powder, not actually making diamond from carbon at STP.
It’s not just the pressure, you have to keep the anvils of the press from melting. IIRC diamond anvils were the norm and likely still are mostly because something better would be likely impossible. It’s hands down the most difficult problem in experimental physical chemistry. Computer modeling is probably the dominant research tool today.
We know far more about other planets than we know about the chemistry of the upper mantle. Almost all the data are simply rocks of different ages formed under different conditions from different ingredients. Walk in a dirty 100 year old chem lab and figure out what experiments were being done by looking at the trash.
I looked at rocks. Not many have ever had the privilege of doing experimental work in igneous petrology.
for one person it’s a diamond, for the next person it’s just another press release…
I didn’t know diamonds grew on plants. ;) All I need now is a money tree!!!! Thanks I’ll let myself out.