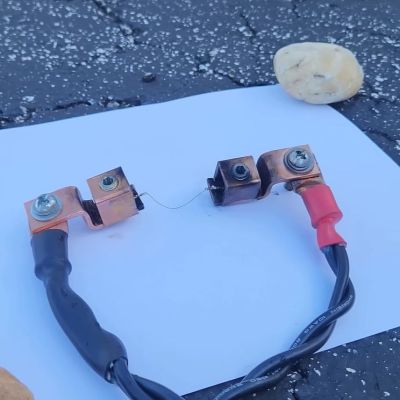
In lieu of high-explosives, an exploding wire circuit can make for an interesting substitute. As [Hyperspace Pirate] demonstrates in a recent video, the act of pumping a lot of current very fast through a thin piece of metal can make for a rather violent detonation. The basic idea is that by having the metal wire (or equivalent) being subjected to a sufficiently large amount of power, it will not just burn through, but effectively vaporize, creating a very localized stream of plasma for the current to keep travelling through and create a major shockwave in the process.
This makes the exploding wire method (EWM) an ideal circuit for any application where you need to have a very fast, very precise generating of plasma and an easy to synchronize detonation. EWM was first demonstrated in the 18th century in the Netherlands by [Martin van Marum]. These days it finds use for creating metal nanoparticles, brief momentary light sources and detonators in explosives, including for nuclear (implosion type) weapons.
While it sounds easy enough to just strap a honkin’ big battery of capacitors to a switch and a piece of wire, [Hyperspace Pirate]’s video demonstrates that it’s a bit more involved than that. Switching so much current at high voltages ended up destroying a solid-state (SCR) switch, and factors like resistance and capacitance can turn an exploding wire into merely a heated one that breaks before any plasma or arcing can take place, or waste a lot of potential energy.
As for whether it’s ‘try at home’ safe, note that he had to move to an abandoned industrial site due to the noise levels, and the resulting machine he cobbled together involves a lot of high-voltage wiring. Hearing protection and extreme caution are more than warranted.
















Joules of energy? Is that like Volts of electricity, Amps of current, Ohms of resistance, Watts of power, or Farads of capacitance?
Joule need to look it up.
Yes, except Volts are potential energy.
Except Volts are differences in electrical potential
Which is potential energy. In order to create an electrical potential, you must separate charges, which requires work – thus energy.
Volts = Joules / Coulomb.
It’s potential, not energy, because the voltage rating tells you nothing about how much charge you moved and thus how much energy you had to expend to do it. Moving one electron requires far more energy than 500 coulumbs, for example.
less energy, I should say*
So not potential energy since it’s potential energy per charge which is something different.
That’s semantics. You can’t have “Volts” without energy being involved, just as you can’t have speed without distance – something must move. Voltage describes potential energy – how much potential energy depends on what charge we’re talking about.
@M that’s essentially the same as saying gravitational potential energy isn’t a thing. Like, there’s a philosophical argument for that, but potential energy is an accepted concept.
Somehow it feels easier to do this accidently, rather than on purpose.
As nice as it would be to avoid manually resetting the trigger, I think I’d be tempted to make something to switch the power, rather than risk burning out some expensive solid state gate, perhaps use a solenoid to set off something like a mousetrap with large contacts, to act as a fast, high current, relay and maybe try putting it under oil or just firing a large spring loaded solenoid bolt at a plate.
It’s an interesting project, sadly I don’t think my neighbors or local authorities would take too kindly to me doing that around here, maybe if I build a Faraday cage around the shed and cover the walls in sound absorbing foam.
This might of been on HAD earlier, but its fun to watch when you have 100 car batteries wired in parallel…
https://www.youtube.com/watch?v=ywaTX-nLm6Y
I seem to recall that 100 car battery affair, that kid is brave, I’ll give him that.
No … volts are not energy.
A voltage isn’t energy directly, but there is energy in the electrical field described by the voltage. Any time there is a voltage difference, there is an energy difference – we just need other information to know how much.
If there is a known unit charge, such as the charge of an electron, then a difference in voltage describes energy directly. Hence why particle physicists use electron-Volts instead of Joules.
Electron-volts aren’t Volts. They are volts times the charge of one electron.
So volts aren’t energy.
I like to use this simple explanation: voltage is like water pressure and current is the amount of water flowing through.
Non-sequitur. You can’t have volts without there being energy; voltage describes a difference in potential energy. Without energy, no voltage.
A Volt is unit energy over unit charge. If we are dealing with charge in the unit amount, we can simplify that Volts = Joules because you’re just going to be dividing by 1. When we are dealing with other amounts of charge, we simply have some other conversion factor where Volts = Joules * N.
It’s just like saying a mile is 1.6 kilometers; just because there is a conversion factor between the two doesn’t mean both aren’t distance. A kilometer and a mile just aren’t the same distance, just like a Volt isn’t necessarily the same amount of energy as one Joule.
Yep, and pressure in a compressible medium, or due to elevation, means potential energy. How much energy depends on how much of the stuff we have.
Just accept that you aren’t quite right. Sure you can’t have volts without energy but you can say that about a lot of things.
You can rearrange speed and distance to be defined by energy just like a lot of things but that doesn’t make them energy itself.
Volts is not energy in the same way distance isn’t energy.
You could say a volt is energy per chargeand distance is just energy per force.
This was all just a silly little correction that you are getting super defensive about.
And what is “energy itself”? Joules? Oh, that’s just kilogram-meter squared by seconds squared. There is no such thing as energy – it’s an intellectual abstraction for the potential of change in a system.
Unless you’re applying Langrangian mechanics instead of Newton’s. Then you’re specifically describing things according to and finding out e.g. which potential paths correspond with your initial and final energy conditions. Then distance IS energy.
https://en.wikipedia.org/wiki/Action_principles#Energy,_not_force
Saying things like “distance isn’t energy” just means you got taught Newton first and then stopped learning about the other formulations of classical mechanics.
In the end, if you want to get philosophical about it, everything reduces to energy.
Even the fact that we have distance at all comes from the fact that space expands due to some form of energy in the universe, without which there would never have been a big bang and we’d still be a point singularity with no size or shape.
😛 Someone forgot they were the one getting philosophical first.
Minor correction of your minor correction blew up into a manefesto on semantics trying to justify your slight mistake.
There’s no mistake. Only a difference in your point of view.
The real mistake is in denying one of two equivalent descriptions of reality.
Hey, ease off… he’s just a dentist
If voltage is potential energy then acceleration is distance
Yep.
You can only get acceleration without actually moving some distance by assuming that no time passes, i.e the difference in time is zero, at which point your definition of acceleration actually breaks down, because you’re just doing division by zero, which is undefined. If no time passes and nothing moves how can there be acceleration? What does it mean to have acceleration of an unmoving object? Sounds like a contradiction in terms to me.
Acceleration implies motion, ergo distance, but it’s not fully defined to say how much distance. That depends on other factors.
Volts are to electromagnetic energy what height is to gravitational energy: you also need to specify how much stuff are you deploying—that’s why it’s called electric or gravitational ‘potential’.
For the former, you need to specify how much charge you have (Energy=VoltageCharge); for the latter, how much mass is lifted (Energy=HeightWeight, where Weight=Mass*Gravity, and Gravity is 9.81m/s2)
Precisely. That’s why I wrote potential energy instead of just “energy”.
When you move something up against gravity, you’re increasing its potential energy, and this is true without the need to specify how many Joules of work we need to spend to achieve this, or even what the gravitational constant g is. Height is potential energy – we just don’t need to specify how much – until such information is needed for further reasons.
For example, I have a pen on my desk and my desk is 75 cm off the floor. If I lift the pen up to a height of 150 cm, how much potential energy does it have?
Answer: twice as much. I didn’t need to weigh the pen or measure the gravitational constant to give you that answer. I’m calculating energy directly based on height, because I simply use the pen itself as the unit mass of this system, so we go from 1 energy to 2 energy. How much “energy” that is doesn’t matter until we start to relate it to something else.
In a similar fashion, when you go to your doctor to have your blood pressure measured, they may take the measurement in millimeters of mercury. How much that is in Pascals, Joules, or anything else is entirely irrelevant to the point. You don’t need to know the density of mercury or how much of it is in the glass tube – all you want to know is how high it rises.
So your blood pressure is measured in millimeters – which seems on the face of it completely daft, but you have to realize, your units are not your measurement. The units are arbitrary – what’s interesting is the changes happening in the system, that’s what you’re really trying to find out.
Gravitational potential energy is usually calculated in its entirety down to Joules. It’s not left as weight or some other hodgepodge.
So of something has 10 joules of gravitational potential energy then that’s that. No need to find the mass or anything else to get energy.
Volts has different energies depending on the charges involved. A volt is more like weight with the finished calculation with the charge being energy.
Volts would be like height and amps would be the weight. Combine the two and you get watts which is the amount of energy. Or joules if you like those units.
Seriously, Steven, using units like that makes you look bad, like you don’t really know what you are talking about.
No reason for that. That’s just convention, and not necessarily even that. The Soviet Union got rovers on the moon and landers on Venus calculating in kilogram-force, not Newtons. The unit is arbitrary. What you’re actually measuring is what matters.
Of course you do. 10 Joules at a certain height depends on having a certain mass in earth standard gravity, which is different in every location on earth. The same mass at the same elevation would have a very different potential energy on the moon. Most of the time, what you’re really interested in, “what if I drop this mass, what happens to that other mass?” and when you run the math, the energy cancels out – it’s becomes a ratio of masses, speeds, distances etc. so it’s just as good you calculate in meter-kilograms or similar.
As opposed to all these other oddball measurements of energy:
https://en.wikipedia.org/wiki/Units_of_energy
This was also an amusing list,
https://en.m.wikipedia.org/wiki/List_of_unusual_units_of_measurement
Or better yet, [ I feel some of these should be used more often. ]
https://en.m.wikipedia.org/wiki/List_of_humorous_units_of_measurement
To avoid mistaking for Joules of Verne or Joules of the Nile.
Is Joule not the SI unit for energy?
Amps of current, Ohms of resistance, etc. are common means of expressing those things in my world.
I guess I just don’t understand what this question is getting at. Is it implying redundancy?
Now, “Amperes of chicken”, I’d trip over something like that for sure. Here in the US, we measure chicken in Buckets.
The Joule is a SI derive unit, not a base unit.
Derived units can be just about anything, although “coherent” derived units use the base units in unit amounts – which means no arbitrary conversion factors to fudge the game.
Everything Hyperspace Pirate does is so sketchy, and I love it.
He does have a talent for it, his videos are a must watch combination of informative wow and OMFG that’s so sketchy plus the narration and graphical style are great too.
This has me thinking: Is there any fast acting chemical reaction (I am guessing that it would probably be an explosive one) that could be used to generate extremely high currents ?
Although thinking about this further, if there has been research done, it is probably secret to prevent the creation of extremely portable EMP devices.
Well, I’ll just leave the link:
https://en.wikipedia.org/wiki/Explosively_pumped_flux_compression_generator
Thanks, that is totally fascinating. But in that instance they are using in effect shaped charges to convert some of the explosive energy into amplifying a preexisting magnetic field through physical compression.
I was thinking more like rapidly acting chemical battery that produces so much current so fast that it self destructs.
There are pyrotechnic batteries that are essentially molten salt batteries activated by a small pyrotechnic charge. Sometimes called thermal batteries. They can put out something like 20-30 kW per kg which is about 2-3 times more than conventional batteries can manage.
They’re mainly used in rockets and missiles where you need a battery that is shelf-stable for 50 years, yet can operate at high power instantly at the push of a button.
Well to create electromagnetic energy you either need to push electrons somewhere or create a magnetic field somehow. Your chemical reaction has to do at least one of those two things. Nuclear explosion create EMP because there are lots of energetic particles that are suddenly moving very quickly through already existing fields inducing energy into all sorts of conductors.
The type of “generator” you linked has historically been used to supply the current needed to vaporize the gold thread in Exploding Wire Detonators in nuclear weapons.
Exploding wire setups were a big deal in the 1940’s and 50’s for observing the spectra of plasmas. By the 1960’s they were a source of entertainment and a fun demonstration in physics departments. Then in the 1970’s if the device had a fast enough capacitor, they became the power source for flash tubes for ruby lasers. Then repurposed to shrink nickel coins.
They were also toned down a little to make camera flash bulbs.
Nothing ‘toned down’ about it. Camera flash bulbs are a chemical reaction, ignited electrically. The flash energy doesn’t come from the electrical source.
The earlier disposable flash bulbs needed a significant electrical discharge on a wire to heat the materials, to set off the reaction, while the later bulbs had a sort of built-in “battery” where you only had to short two contacts together and that would cause enough heat to start the reaction.
You’re on the right path to that glow-in-the-dark antenna y’all wanted… just dial it back a little.
Nah.
Remember someone writing about Creating EMP’s Using exploding wire methods. interesting….
So funny, I came here to write about exploding wires for EMPs. I never thought of other uses than generating huge voltage spikes.
After watching the video, a couple of thoughts came to me:
First… this guy MUST hook up with the Slow-Mo Guys. I think the annihilation of wire segments or aluminum strips at 10’s of thousands of frames per second would be fascinating to see.
Second… on his home-brew contactor… an obvious improvement would be to solder/braze a couple of silver “mercury” dimes to the contact faces. In a machine that dumps thousands of amps, milliohms matter.
Another thought would be to replace flat-surface contacts with a copper cone-and- socket arrangement. A cone and socket maximize contact area (minimizing contact resistance), it would be self adjusting for wear, and to some extent, the inherent wiping action would make it self-cleaning. Rather than drive the cone into the socket with a lever handle, a much better arrangement would to use a stiff spring, cocked before firing, and released with a pin. The pin, in turn, could be actuated from a distance by a small solenoid.
The cone and socket could also be silver-plated.
As the victim of an American engineering education, Joules are a non-intuitive unit for me, and I had to look up the conversion. 1 Joule = .74 ftlbs . So 4000Joules = 2950 ftlbs, or the kinetic energy accumulated by a 500 lb weight dropped about 6 ft.
For gun people, the muzzle energy of a .50 cal round is around 13,000ftlbs. The .308 he compared the noise to, about 2600 ftlbs, and a .223 AR-15 round around 1200 ft*lbs. So even if the watermelon explosion seemed a bit anemic, he’s dealing with significant energies here.
Recallling my factory electrician days, I do not regret my habit of turning my head away whenever I reset a switchgear.
A Joule is roughly the potential energy of a small apple falling on your head from 1 meter above.
4000 Joules is a wheelbarrow full of apples being dumped on you from a balcony one floor up.
It’s also just under one (nutritional / kilo) calorie. Enough energy to raise the temp of one liter of water by one degree C.
https://en.wikipedia.org/wiki/Calorie
Now just think of how much power there is in a candy bar!
To clarify, one Joule isn’t enough to heat a kilogram of water by 1 C. 4184 Joules is.
A person consumes about 8-9 Million Joules per day. Your car uses about 2000 Joules every kilometer you drive. The Joule is one of those metric units that are inconveniently small to describe everyday things in any way you could easily relate to, because anything meaningful is just really a big number. That’s why everyone uses kilowatt-hours (not a SI unit) or calories, or BTU instead.
Actually, if you drive a kilometer in a car that goes 5 L/100km, you’ve consumed 5 x 8760 Wh / 100 = 438 Wh of energy per kilometer. A watt-hour is 3600 Joules, so you’ve actually consumed 1,576,800 Joules of energy.
As is said, the metric system is easy with no weird conversion factors between units – I’m just too dumb to use it.
Gonna need another generator!
https://youtu.be/wrFlAkLbvfw
One day in physics class in first year university, 40 years ago, the professor rolled a rather large capacitor out on stage. A wire was attached to one terminal. The other end of the wire was attached to a plastic rod about a meter long. The rod was used to stretch the wire across to the other terminal. After a tremendous bang, the wire was gone. Vanished. Afterwards, a resistor was connected between the terminals, to prevent any unintentional charge build-up.
I still clearly remember that demonstration…
It is sobering and very sad to see such discussion on units here. It’s not a matter of debate. If you don’t know what it is, go look it up. If you think you know, go look it up before making an ass of yourself here.
It’s embarrassing commentary on our educational system to see such nonsense, especially in an ostensibly tech-literate forum like this.
It’s not that bad. Just pedantic nerds showing off their lack of social skills.
So it’s a fuse?
No, usually we prefer fuses to just melt. Exploding is undesirable. Its really a matter of speed.
And, yet, exploding fuses are very much desired in high voltage high power circuits (think big overhead power lines). The gas generated blows the arc out.
Used to do exploding wires with ~500 µF 2,000 VDC capacitor bank, which was original used for a homemade ruby laser flash lamp supply in 1970’s. The switch was an air-gap thyratron — just three 8-32 bolts inside a block of plastic. The third electrode was attached to the output of a car spark coil, in turn triggered by a small capacitor and small (7 amp) SCR.
Actually, I still have that device, though the electrolytics would need slow, careful reforming. BTW, for series electrolytics, there should be equalizing resistors across each, or, after repeated firings, there will be large voltage variation due to parts tolerance, blowing up one of the caps, rather than the wire.
I’d like to think that this would be done in a glass jar or similar protective envelope. The “exploding” material could be damaging to tissue (I’m thinking eyes). A colleague of mine apparently demonstrated this inside a glass jar in a museum years ago, said it was quite impressive :-)
Nice… P_O_P
Can this be, in a vacuum chamber, a nice way to deposit metal on stuff?
Funny, I was thinking just the opposite.
Containing pressure waves = shrapnel if the container fails. Whereas if you get some copper dust on you, it’s no big deal, and they’re likely to decelerate within a foot or so.
I think the glass jar will not resist to the shockwave (but it may hold to the final equalized pressure). So if you suround the wire with rockwool to form a kind of silancer, the jar may hold.
Probably not. The sound you are hearing is air rushing into space being vacated by the vaporizing wire and super fast expansion caused by plasma heating. This is happening faster than the speed of sound so the waves pile up and hit you all at once. This is a pressure wave and will act on whatever tries to containing it. Padding would slow it but ultimately the pressure wave is still there. Just like water hammer in a pipe.
The glass will contain the pressure waves that are likely to damage your hearing first.
Not to mention that some of the bits might no vaporize completely and end up with a millimeter sized wire fragment lodged in your eye.
The aluminum foil oxidizing is cool. Until I watched a video on thermite I don’t think I understood how rusty the surface of a metal like aluminum is. If iron was the same I’m guessing the average person wouldn’t know that it’s pure colour was not orange/brown. The aluminum oxide layer is extremely thin but prtect the bulk of aluminum from oxidizing in any appreciable time, which is why a thermite is so shelf-stable.
In this case the aluminum plasma becomes oxidized soon after the aluminum ions cool back into atomic aluminum since the whole mess is surrounded by air.
Back in the late 1970’s I was part of a company that assembled MSI6800 kits for re-sale. The PCB’s were not of the best quality and particularly the memory boards would fail under test with either an address of bit fault. A quick check with a meter often showed a pair of shorted tracks. Visually you could spend hours looking for a hairs-width copper whisker. To save time I came up with a solution which became known as “cappy job”. I took a large value electrolytic capacitor and charged it to 5 volts. A pair of test probes were applied to to each of tracks involved and, after a quick “crack” the problem was solved. I never lost a memory chip and the boards ran flawlessly after “treatment”. As the chips were 5 volt logic I figured that any input protection diodes would not be stressed by the application.
What was the current rating of those diodes? IMHO the only thing that saved those chips was the fact that the copper whisker was a lower resistance path and the bulk of the energy was discharged there.