Sometimes you come across a purported scientific paper that makes you do a triple-check, just to be sure that you didn’t overlook something, as maybe the claims do make sense after all. Such is the case with a recent publication in the Langmuir journal by [Budlayan] and colleagues titled Droplet-Scale Conversion of Aluminum into Transparent Aluminum Oxide by Low-Voltage Anodization in an Electrowetting System.
Breaking down the claims made and putting them alongside the PR piece on the [Ateneo De Manila] university site, we start off with a material called ‘transparent aluminium oxide’ (TAlOx), which only brings to mind aluminium oxynitride, a material which we have covered previously. Aluminium oxynitride is a ceramic consisting of aluminium, oxygen and nitrogen that’s created in a rather elaborate process with high pressures.
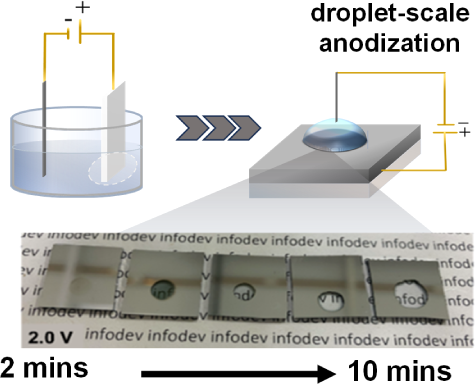
In the paper, however, we are talking about a localized conversion of regular aluminium metal into ‘transparent aluminium oxide’ under the influence of the anodization process. The electrowetting element simply means overcoming the surface tension of the liquid acid and does not otherwise matter. Effectively this process would create local spots of more aluminium oxide, which is… probably good for something?
Combined with the rather suspicious artefacts in the summary image raising so many red flags that rather than the ‘cool breakthrough’ folder we’ll be filing this one under ‘spat out by ChatGPT’ instead, not unlike a certain rat-centric paper that made the rounds about a year ago.















Perhaps ChatGPT watched too many star trek movies… Scottie and the mouse was quite amusing.
I’ll agree that the cover image looks a bit odd, but I don’t think the chemistry is too weird. They are turning a 100 nm film of aluminium (so about the same thickness as gold leaf) into its (hydr)oxide, which is transparent. It’s not turning a large chunk of aluminium transparent, or into some nitride (which is never mentioned in the paper?), just a thin layer on a glass slide.
Electrochemical transformation of aluminum to alumina is indeed industry standard. The paper is not shady, it is just not very exciting. That is why it appeared where it did.
It would have been nice if they hadn’t cropped out all the edges of the glass slide in their photograph.
do they prove that it is not just hole in alu layer eaten by acid? :D
[Sticks finger through hole and wiggles it around]
“Well the good news is that it IS see-through, but I think we need to revise our paper unfortunately…”
If this is legit I’d assume there would be actual applications like scratch-proof lens coatings for small optical sensors
I wonder if it could be used for cheaper and more durable phone screens.
Synthetic sapphire is used for iPhone screens but I wonder if it’d be possible to get the same scratch resistance by coating normal glass with aluminum then converting it using this method.
All iPhone screens are Gorrilla glass…the only place they sometimes use sapphire are the rear cameras
Yeah but with a cheaper process they would definitely start using this for larger surfaces is the point. A bit like how regular aluminum went from being an expensive and exotic material to hardly worthwhile to pick up and recycle after a new process was discovered to produce it.
My mistake. I recognized Apple used it but don’t pay enough attention to them to get the details correct.
I love the irony of this comment, this is making sapphire
Where is the irony the comment is recognizing that it makes sapphire and might be useful for possibly bringing down the costs for additional scatch resistance on screens.
I’m no materials scientist but I’m fairly sure glass is harder than aluminium by a long way already.
Yes, but aluminium oxides are very hard – Alumina has a mohs hardnes of 9. Aluminium Hydroxid on the other hand has seemingly only a 3, between a fingernail and copper.
It looks like the article is making sapphire. Sapphire (corundum, which is ruby or sapphire depending on the doping) is basically the second hardest (least scratchable) material we have, behind diamond. This would be a fantastic method for making something more scratch resistant than traditional glass. The hardness of this material is probably similar to gorilla glass, but it’s also a very simple process.
that is very thin leaf of Al and this is not like free article all about article is suspicious maybe those just holes in leaf .
What exactly is the big deal about ‘transparent aluminium oxide’? Synthetic sapphire is aluminium oxide and is transparent from the UV to the near IR. Among other things it’s used to make optical windows, including covers for camera lenses and fingerprint readers.
Hand in your nerd card, and then read the very first comment.
TL;DR film reference.
I’m quite aware of the film reference, having been a ST fan since the original series back in the 60s. My point was: what is it about making transparent aluminium oxide that is so significant that it warrants a (presumably peer-reviewed) publication, when transparent Al2O3 has been made commercially, in large quantity, for decades?
There are lots of ignorant trekkies that go nuts whenever the word “transparent” appears next to the word “aluminum” while failing to understand that aluminum and aluminum oxide are about as interchangeable as steel and rust.
They usually aren’t ignorant or failing to understand that… They just are able to suspend disbelief for a sci-fi show
Okay, I don’t get it. This paper might be questionable (I have no opinion), but “transparent aluminum” is an actual, real material, manufacturable on Earth with 21st Century technology. Last I heard it simply wasn’t cheap. We can debate it until we are blue in the face, but the proof is in reproducing the results.
Simply put, when it says Al, I expect Al, not Al2O3 or Al(OH)3.
Not only is it accurate to make the distinction, but there’s you “can” get metals to be transparent. The catch is that it requires < 20 fs pulses of 1E+17 W/cm² x-ray intensity:
https://phys.org/news/2024-07-copper-transparent-european-xfel-exotic.html
As far as I understand the experiment, the foil is not vaporized. Of course it’s a curious super fast electron dynamics experiment and nothing that will lead to a new smartphone screen, but I don’t make the rules.
TIL ‘Synthetic sapphire is aluminium oxide’. There are a class of instruments which are driven underground for in situ sensing of environmental contaminants(often petroleum, but also other compounds). They project UV light through a sapphire window onto subsurface soils, and register the subsequent fluorescence of the contaminants. The sapphire window is a key element as it needs to endure considerable pressure and abrasion while passing light over a broad band.
Well it’s not a Chinese study so that’s promising at least
I bet Scotty is proud.
“The Endochronic Properties of Resublimated Thiotimoline”.
Isaac Asimov came in first.
You can’t make it if you don’t talk to the mouse (refer to Scotty comment above)
Yes this research article definitely stirred up questions. Some good ones in this thread. Here’s a bit more from the authors that was informative: https://www.nelsonpub.com/cms/dfx/opens/article-view-dfx.php?nid=4&bid=1509&et=featurearticle&pn=03
Honestly I found it inspiring, I will definitely see if I can reproduce it myself to some degree.
Anyone know where to access the full text without a paywall?