There’s a classic grade school science experiment that involves extracting juice from red cabbage leaves and using it as a pH indicator. It relies on anthocyanins, pigmented compounds that give the cabbage its vibrant color but can change depending on the acidity of the environment they’re in, from pink in acidic conditions to green at higher pH. And anthocyanins are exactly what power this unusual kinetic art piece.
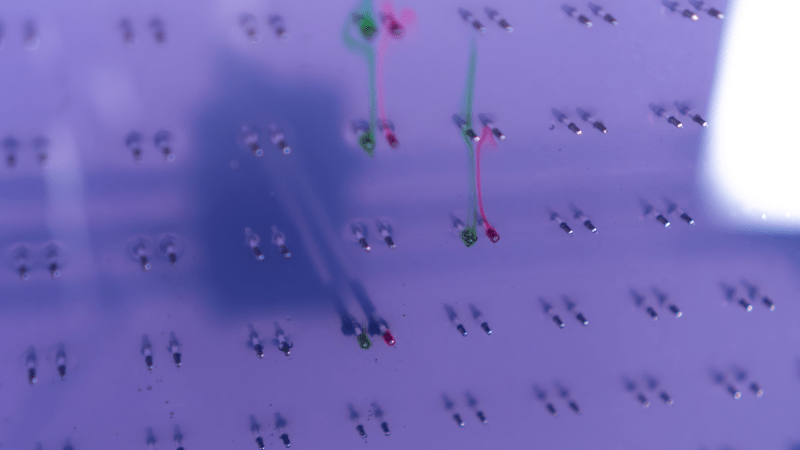
Even before it goes into action, [Nathalie Gebert]’s Anthofluid is pretty cool to look at. The “canvas” of the piece is a thin chamber formed by plexiglass sheets, one of which is perforated by an array of electrodes. A quartet of peristaltic pumps fills the chamber with a solution of red cabbage juice from a large reservoir, itself a mesmerizing process as the purple fluid meanders between the walls of the chamber and snakes around and between the electrodes. Once the chamber is full, an X-Y gantry behind the rear wall moves to a random set of electrodes, deploying a pair of conductors to complete the circuit. When a current is applied, tendrils of green and red appear, not by a pH change but rather by the oxidation and reduction reactions occurring at the positive and negative electrodes. The colors gently waft up through the pale purple solution before fading away into nothingness. Check out the video below for the very cool results.
We find Anthofluid terribly creative, especially in the use of such an unusual medium as red cabbage juice. We also appreciate the collision of chemistry, electricity, and mechatronics to make a piece of art that’s so kinetic but also so relaxing at the same time. It’s the same feeling that [Nathalie]’s previous art piece gave us as it created images on screens of moving thread.















Someone should make a clock out of that
*I should make a clock out of that
*I already have too many projects
*Someone should make a clock out of that
I absolutely love this
I’d imagine you can make primitice images in rbg doing this in cells all at once (or timed) to create a, say, 32×32 image ?
So much art. So much wow.
I assume the reaction products are both less dense than the baseline solution, which is why they form trails upwards during the energization. For some reason I find that surprising; I guess I figured one of them would end up heavier since the oxygen added to the oxidized product would either be heavier or lighter than what it replaced or joined too (depending on the chemistry). But I guess not. Maybe the products are getting heated by the process and are thus less dense? Some many questions. And I want to watch it for longer to get into the slow mental space I’m assuming it would elicit. Very cool.
Oxidation in this case is the loss of an electron, not gain of oxygen. An interesting review of anthocyanin chemistry (in the professional literature, but there are some nice chemical structures to showcase what is happening) is here: https://pmc.ncbi.nlm.nih.gov/articles/PMC7504512/ . I am speculating as to why they would both float up, but it may be due to the potential used in the electrolysis. If it is just high enough to convert water to oxygen at the anode and hydrogen at the cathode, then microbubbles of gases might be formed, and these bubbles are causing the convection. One can see some bubbles forming on one of the green-colored electrode in the cover picture. We’d need to learn more about the applied potentials to confirm.