Although often glossed over, the human liver is a pretty amazing organ. Not just because it’s pretty much the sole thing that prevents our food from killing us, but also because it’s the only organ in our body that is capable of significant regeneration. This is a major boon in medicine, as you can remove most of a person’s liver and it’ll happily regrow back to its original volume. Obviously this is very convenient in the case of disease or when performing a liver transplant.
Despite tissue regeneration being very common among animals, most mammalian species have only limited regenerative ability. This means that while some species can easily regrow entire limbs and organs including eyes as well as parts of their brain, us humans and our primate cousins are lucky if we can even count on our liver to do that thing, while limbs and eyes are lost forever.
This raises many questions, including whether the deactivation of regenerative capabilities is just an evolutionary glitch, and how easily we might be able to turn it back on.
Regenerating Vs Repair
Even in the absence of a regenerative ability, animals can heal injuries, which generally means the growth of fibrous tissue called scar tissue. This can be observed very clearly on our skin, where certain old injuries tend to remain clearly visible as the scar tissue replaces skin tissue. While made of the same collagen protein as skin tissue, the fiber organization is different and serves no real purpose beyond sealing up a lesion. Scar tissue can form elsewhere in the body too, where it can impede function, as in the heart and lungs.
Both regeneration and repair are a form of healing in an organism, but only the former restores the original functionality, whereas the latter is the biological equivalent of slapping on a duct tape patch and calling it good. This ‘repair’ outcome is effectively an incomplete regeneration process, where instead of the affected site creating the conditions for normal growth – leading to a good-as-new result – you only get the basic scaffolding while certain biochemical pathways are never or insufficiently activated.
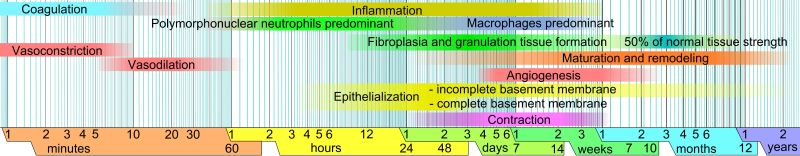
Although it’s often said that the human liver is the sole organ capable of regeneration in our species, it could be argued that our blood vessels are a much better example of regeneration. Within minutes after receiving a cut or bad scrape, any damaged blood vessels are plugged and macrophages along with other specialized cells begin to move into the area as the inflammatory phase begins.
At the end of this phase, angiogenesis commences, which involves existing blood vessels growing new blood vessels into the affected area. In a developing embryo, this is the stage that follows the earliest development of the initial blood vessels through vasculogenesis. In this regard, blood vessels can be said to regenerate themselves in the case of injury. They can also expand into tissues where e.g. hypoxia conditions are present, which triggers the hypoxia-inducible factor (HIF) signaling path.
In the case of wound healing this signal path is stimulated due to the hypoxia condition that exists at the injury site. Although the HIF-related HIF-1α subunit is constantly expressed, oxygen-dependent prolyl hydroxylases (PHDs) normally degrade it and thus downregulating the further responses down this chain.
Another aspect here is the re-epithelization, whereby surrounding skin cells move towards the wound, multiplying until the signals that induce this growth are downregulated below a critical threshold. Based on research the same HIF pathway is implicated here. For example, in a 2015 study in Science Translational Medicine Yong Zhang et al. reported that forced upregulation of HIF-1α was able to induce full regeneration of a hole punched in the ears of mice who normally just show scarring.
This indicates that boosting the HIF signaling pathway might be a viable way to prevent scarring and induce full regeneration of certain types of wounds to the skin.
Blastema Limbo

The HIF signaling pathway is an example of a basic regeneration pathway involving a single organ (i.e. the skin). Things get more complicated when there’s the removal of something to the extent of a limb. Among mammals regenerating ability is limited, with some species like rabbits still possessing the ability to regenerate holes in their ears while other species, including humans, are not creating the requisite blastema of undifferentiated cells after an amputation.
The axolotl is one of the most studied species when it comes to tissue regeneration. Similar to other salamanders they possess a remarkable ability to regenerate many parts of their body, with the axolotl capable of regenerating their limbs, gills, eyes and parts of their brain. Although annelids (segmented worms) and echinoderms like starfish are capable of even more extreme forms of regeneration, axolotls are significantly more akin to us mammals than either of those.
Incidentally, similar research in fruit flies (Drosophila melanogaster) has led us to the highly conserved Hippo signaling pathway. This particular signaling path is essential in determining how big an organ is supposed to be, such as when a human liver is chopped up in vivo and has to regrow back to its original size.
New Limb Cap
When an axolotl suffers severe injury like the loss of a limb or a gill, the surface where the amputation occurred gets covered up by epidermal cells, forming the wound epithelium (WE). This is the point where for human and other mammals the process pretty much ends with a stump covered up by skin. In the case of the axolotl, however, this WE keeps gathering epidermal cells, forming the apical epithelial cap (AEC).
Inside this AEC the tissues then undergo dedifferentiation into a blastema – led by signals from macrophages – effectively resetting the tissues here to a much earlier, embryonic state of development. Under the influence of Hox genes which regulate the body’s layout, the AEC subsequently grows as it would have done previously with the very young axolotl until the entire limb, gill, eye, etc. has been regrown.
The trick is thus to take these identified signaling pathways, establish in how far they have been preserved in other animals – like us primates – and whether we can easily re-enable them in some way, whether permanently or temporarily. After all, it worked once when we were still embryos, ergo by resetting the cellular clock on part of our bodies it would simply run through the same biochemical steps again.
Still A Lumpy Road Ahead
Of course, this involves developmental biology, biochemistry and genetic research, meaning that clear answers are rarely found and require immense amounts of research and study to unravel how all of these signaling pathways work, while maybe finding a few more ones along the way. The upshot of course is that the field of regenerative medicine can have massive implications for human health, ranging from the ability to treat many (genetic) disorders related to faulty signaling pathways to the ability regrow limbs, eyes and more.
It’s likely that regenerating skin and directly related tissues in human patients will be one of the first widescale applications of these findings, with recently Weifeng Lin et al. publishing a study in Science involving regrowing a damaged outer ear (pinna) of mice and rats through the addition of retinoic acid (RA), a key element in embryonic development. Specifically they identified that in non-regenerative species of rats and mice the Aldh1a2 gene was not expressed as much as it was in species who do regenerate, which reduces the amount of available RA from the retinaldehyde precursor.
Although there’s a lot that can be said about the pros and cons of turning back on genes that haven’t been active since we were either an embryo or a still-growing-child, understanding these biochemical pathways offers us the prospect of bypassing them in order to restore that which once was thought to be lost forever. Even if we won’t be regrowing limbs yet next year, we might be giving people back their pinna, digits, faces and erase old scars before we know it.
“Closeup of Axolotl in Hand” by [Yaiol AI]
“Purple Tropical Axolotl” by[ Raphael Brasileiro]
















Here here for regeneration of the hair cells in hearing. I heard for 30 years or more about being able to do this in human subjects. Chickens do it!
If you were trying to use the English expression, often used in parliament, I have to point out it’s ‘hear! hear!’ not ‘here here’.
He can’t hear. That’s why he’s here.
Usually sounds more like hrrrrrrgh hrrrrrrrgh’k
I was thinking about why Americans often get it wrong, because ‘hear hear’ also seems so much more logical to me. Then it came to me that it’s sort of the opposite of the expression ‘there there’, and perhaps that’s why there is a tendency to think it’s ‘here here’ :)
I guess the US version (of the correct one) would be the expressions ‘listen to the man’ if someone says something you agree with and people should hear it, which relates to ‘hear! hear!’
“Hear, Hear” was used in America long ago, although certain states and federal courts are announced with “Hear Ye, Hear Ye!” and even the Anglo-Norman “Oyez, Oyez, Oyez!” is still in use. “Listen to the man” kindof assumes Americans didn’t use the same phrase, and while it’s considered old-fashioned, in the US we still verbally understand and say “hear, hear!” I had given an alternative explanation why Americans might spell it “hear”, but was censored by Hackaday’s “nonce” algorithm.
This topic is absolutely fascinating, and I really enjoyed the article. Plenty of SF stuff about “regen tanks” etc. and I can see here the seeds of making that a reality. That said, it’s freaking complicated, so comments like this:
make me tweak a lil bit – there are lots of factors involved, and usually traits get dropped for a reason. I find it perfectly plausible for instance that the regeneration traits could negatively affect complex brain structures that need to stay how they are in order to have long-term memory. So it’s plausible to me that we would need to grow limbs in a tank and then reattach, or maybe hook up an alternate blood supply that provides the regen signals to the limb but not the rest of our body, etc.
That said, still reaaaally coool :)
That comment was naturally written tongue-in-cheek, as nothing in biochemistry or evolutionary/developmental biology is ever ‘easy’ or ‘straightforward’, but it makes sense as a reasonable starting question at least :)
Most likely it’s just hard to avoid creating cancer when regenerating mammal parts. I’m not saying it’s entirely impossible for sure or that we shouldn’t be working on it. Just that is probably why we lost the ability in the first place and that will be the biggest hurdle in medically regaining it. It will probably be a very very high hurdle, but totally worth it in the end.
Agreed, the lack of redundant data to resist cancer compared to species with more advanced regeneration stands out starkly.
I work with similar regulatory networks in mosquitos on their transcriptome regulation. Stress signal pathways have some really powerful activities in some species that confound some traditional ideas on how transgenes interact with intercellular systems. Cells will compete with eachother and overtake roles if they can’t handle the overproduction of a marker gene, triggering nearby cells to overtake their duties.
I think one of the best review scientists on these sorts of intercellularly directed repair has been Michael Levin and his modeling of that communication in action during repair and development. He’s covered a lot of subjects that build up a larger model of scaling “intelligence” (dont like his terminology, but there seems to be a goal state that’s directed by intercellular activity) across different fields. Since I’m on the edge of transcriptomics and intercellular systems, I can’t really say much on his computer science theories but his experiment with axolotl limb transplants and the reprogramming of a tail into an arm shows an astonishing flexibility and plantlike development I wouldn’t think an adult animal would preform. I’m lucky enough to have received an axolotl arm when I was at a conference in Toluca (they rip them off all the time in their juvenile stages allegedly, and I received it as a small gift from a student at the university) where there are still some wild populations.
There is a larger system in regeneration that really needs to be explored further, but the potential for med hacking has never been so bright IMO.
Very cool stuff, I’ve been slowly following the advancements and while some of it plods along, the variety and complexity of these interactions is bewildering enough it’s no surprise.
Other vertebrates are useful in this regard, and I seem to remember alligators being studied for limb regeneration (crocodilians in general have amazing immune systems and regenerative capabilities, although they paradoxically don’t seem to handle gut parasites very well).
There are also the occasional freak humans that grow back much more of their missing digits than they should be able to, so they would probably be worth tracking down for genetic tests. (Assuming you could entice anyone to self-report in order to bypass medical privacy regulations, and assuming their medical records, once released, supported their claims.)
The reason why land animals don’t grow back limbs has probably something to do with the amount of radiation you’re exposed to when you’re not living most of your life under water.
Regeneration is kinda like controlled cancer. If there’s a tiny mistake, the cells won’t know to stop.
Hey! We all could use an extra pair of hands sometimes!
After a certain age, I bet many would take risk of super cancer if it meant they could regenerate back to youth.
At least cancer is something we can fight even if only just a little. If we could fight limb loss, aging, and other deterioration then I think it’d be worth it.
“Fighting” a full body cancer might be a little harder than you imagine.
Might not occur like that though and I’m sure it’d still find popularity with the elderly or heavily crippled as a last hurrah at worst.
At best the cancer rate would be low and local.
The main point is that if we re-engineer our cells to be able to regenerate lost limbs, we’re also engineering them to be prone to cancer, so while you might get your finger back you also risk dying in a couple years.
Less so if the limb or organ is mostly pre built, then grafted. Given mammalian susceptibility to cancer this is likely the only reasonable approach. This also opens up the possibility of accelerating the process with partially manufactured bone and tissue.
It took me about 15 years to grow my hands to about the size they are now the first time. So if I lose one and it regenerates back, would it take another 15 years? Walking around with a tiny hand just like Deadpool except stuck that way for years! But still better then no hand at all.
Speaking as a “Boomer”? Oh to have a way to replace the now absent cartilage for my knees and some arthritic damages starting elsewhere! I’m well beyond any syn-visc sort of treatments at this point. Just not wanting to have the knees cut out yet, in case someone is close to needing a patient, to try some promising idea on.
I own an Axolotl, when we bought it was missing fingers on one hand due to being attacked by one of its siblings at the store. They grew back quickly but he now has different numbers of fingers on each limb. They’re amazing creatures (just a shame they’re dumb as pudding)
All that regeneration is energy intensive, if they had a higher level of social intelligence it might not be as thorough.
Maybe they are dumb because their brain keeps regenerating and returning back to “defaults” instead of pruning connections and making patterns.