There was a recent recall of so-called ‘radioactive shrimp’ that were potentially contaminated with cesium-137 (Cs-137). But contamination isn’t an all-or-nothing affair, so you might wonder exactly how hot the shrimp were. As it turns out, the FDA’s report makes clear that the contamination was far below the legal threshold for Cs-137. In addition, not all of the recalled shrimp was definitely contaminated, as disappointing as all of this must be to those who had hoped to gain radioactive Super Shrimp powers.
After US customs detected elevated radiation levels in the shrimp that was imported from Indonesia, entry for it was denied, yet even for these known to be contaminated batches the measured level was below 68 Bq/kg. The FDA limit here is 1,200 Bq/kg, and the radiation level from the potassium-40 in bananas is around the same level as these ‘radioactive shrimp’, which explains why bananas can trigger radiation detectors when they pass through customs.
But this event raised many questions about how sensible these radiation checks are when even similar or higher levels of all-natural radioactive isotopes in foods pass without issues. Are we overreacting? How hot is too hot?
Healthy Radiation, Normal Radiation

Ionizing radiation from nuclear sources forms both an unavoidable and an essential part of food safety. The practice of food irradiation involves exposing food to gamma rays in order to destroy anything that is still alive in it, like bacteria and other potentially harmful microorganisms. Much like heating with pasteurization and similar practices that aim to wipe out these microorganisms, this can render food safe for consumption for much longer than would otherwise be possible.
Whereas food irradiation does not actually introduce radioactive isotopes to the foodstuffs, these isotopes can still enter prospective food in other ways. Long before these infamous Indonesian shrimp – likely prawns – found themselves post-mortem on their way to the US, these critters were either happily galivanting about in the Pacific Ocean or less happily stuck in a shrimp farm, doing all the things that pre-mortem shrimp do. This includes consuming a lot of shrimp food, starting with plankton and moving up to worms, bivalves and other crustaceans as they mature.
All of these food sources along with the water that they live in contain some level of radioactive isotopes, ranging from the uranium-238 that’s plentiful in seawater, to tritium from atmospheric sources, and manmade isotopes like cesium-137 from nuclear weapons testing. Most isotopes, including Cs-137, do not bioaccumulate: in humans Cs-137 has a biological half-life of about 70 days . This suggests that this particular batch of whiteleg shrimp ingested some kind of relatively Cs-137-rich food shortly before harvesting.

The Pacific Ocean area was a particularly prolific area when it came to nuclear weapons testing, with of the worldwide approximately 2,121 tests so far the US and France detonating a significant number in the Pacific. Tests such as the 15 megaton Castle Bravo experiment featuring the ironically named SHRIMP device, which significantly raised the amount of carbon-14 (C-14), Cs-137, and strontium-90 (Sr-90) in the region. Due to its bioaccumulating nature, Sr-90 with its 29-year half life poses a particular risk, while C-14 with its 5,700-year half life is generally deemed of no consequence, on par with the normal intake of potassium-40 as both isotopes behave in a very similar way in the body.
Although the amount of Cs-137 from these tests has reduced significantly due to natural radioactive decay, this provides one potential path through which these and many other isotopes from both manmade and natural sources can find themselves inside small crustaceans prior to their untimely demise at the hand of bipedal primates with an appetite for seafood.
The one question that remains here is how we can know that a certain amount of an isotope per kg of foodstuff is too much for human consumption. How dangerous is the radioactive potassium-40 in bananas really?
Setting Limits
We earlier listed the FDA’s 1,200 Bq/kg as the limit for the Cs-137. A radioactive source rates one Becquerel if it undergoes one disintegration event per second, and dividing this by the weight gives a rough measure of radiation density. But all decay biproducts aren’t created equally. If we look at the FDA guidance documents pertaining to radionuclides in imported food, we can see that this listed limit pertains to the so-called Derived Intervention Levels (DILs), superseding the older Levels of Concern (LOCs). The same document lists the DILs for other isotopes, including:
- Sr-90 at 160 Bq/kg.
- Iodine-131 at 170 Bq/kg.
- Cs-134 + Cs-137 at 1,200 Bq/kg.
- Pu-238 + Pu-239 + Am-241 at 2 Bq/kg.
What these isotopes have in common is that they are generally only produced by artificial sources, while omitting a very common natural isotope like potassium-40 (K-40) which only forms the third-largest source of natural background radiation after thorium-232 and uranium-238. Since K-40 is readily present in soil and anywhere else that other potassium isotopes are present, it’s practically unavoidable to consume significant amounts of K-40 each day, regardless of whether you’re a crustacean, plant or mammal.

K-40 is both a beta and gamma emitter, with approximately 140 grams of it present at any given time in a 70 kg adult human body, where it is responsible for an approximate constant 4,000 Bq of radiation.
Despite the long half-life of 1.248 billion years, K-40’s prevalence makes up for this sluggish nuclear decay rate, with around 4,000 of such disintegration events happening inside an adult human body each second, as a K-40 nucleus decays into either argon-40 or calcium-40 via gamma or beta decay respectively.

We can contrast this with Cs-137’s 30 year half-life and somewhat similar decay into barium. Nearly 95% of Cs-137 nuclei decay into the metastable barium-137m via beta decay, before decaying into the stable barium-137 via gamma decay. The remaining 5% decay immediately via beta decay into this stable nucleus.
The much shorter half-life and primary gamma decay route make Cs-137 significantly more radiologically active than K-40. Yet while more gamma radiation may sound worse, one has to remember that the biological impact for radiation exposure once ingested is flipped around. For example, while the very powerful alpha radiation is luckily stopped by the top layers of our skin and dissipates its energy mostly in dead skin cells, you don’t want the same to happen to living cells like the inside of your lungs or various other soft issues, with alpha radiation absolutely cooking the nearest layers of cells.
This is where gamma decay ironically helps to distribute the radiation exposure from Cs-137 somewhat, while also complicating the comparison with K-40, as that isotope decays mostly via beta decay and thus can potentially do more damage per event to local tissue as beta radiation does not travel as far through the body.
Overabundance Of Caution
The American Nuclear Society (ANS) article on the “contaminated shrimp” event probably puts this event in best context. Normally shrimp from the Pacific region contains some level of Cs-137, but these recent batches caught the attention at the importing ports due to a 100x higher level of Cs-137 than normally seen. That sounds like a problem, but it only places the shrimp roughly in line with bananas.
A 2023 study performed in Poland found that of animal products produced in that country, cattle muscle tissue showed Cs-137 levels up to 23.5 Bq/kg (wet weight), sheep nearly 50 Bq/kg, and in wild game animals some muscle tissue scored well over 4,000 Bq/kg. All of which place these commonly consumed animal tissues well above the typical value for Indonesian shrimp, and either in the ballpark or significantly above that of the ‘contaminated’ shrimp.
Threshold Models
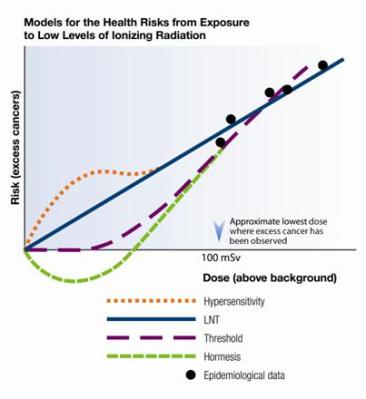
Government regulations pertaining to radiation exposure are most often based on the linear no-threshold (LNT) model, which extrapolates down from very high radiation doses where we can measure the damage more easily. But it does so linearly, making the assumption that ten multiple small doses, even if they are spread out over time, are equivalent to one exposure that is ten times as strong.
Recent studies have suggested that below 100 mSv there are no observable effects, which suggests that a model that incorporates a threshold might make more sense for radiological contamination of food.
The National Academy of Science report on low levels of radiation from 2005, on which most of the US regulations are based, at the time rejected the threshold model due to insufficient evidence. They also cite studies where very small doses are claimed to have negative effects on children still in the womb, suggesting that the lower threshold may not be uniform across different populations.
Even if a lower threshold does exist, and there is an increasing push by scientists for moving past the LNT, establishing the exact value for this threshold is difficult. Below a certain dosage, there just isn’t significant epidemiological data. You cannot prove a negative: “below this level there will be no increased risk of cancer”. One can only say that no excess risk was detected in this or that particular study.
Add in sensitivity about manmade radioisotopes in drinking water, food, and anything else that is sold or presented to the public, and most governments take the LNT approach even if it is likely to be very conservative. And that, in short, is why we got a ‘radioactive’ shrimp recall, when eating that banana might arguably be more hazardous for you.
















Even if the LNT model is true, below a certain point where we cannot even measure the effect because it’s so small that it’s hard to show even in large multi-decade studies, the question becomes does it even matter?
The underlying question is, why should we mandate absolute zero risk? We accept much greater risks elsewhere, so what makes radioactivity special?
Possible birth defects, sometimes rather horrifying. Don’t see the effects soon after exposure like with consumption.
Again, if it’s so rare, is it worth the trouble?
We have medicine with possible side effects that include birth defects. Not as bad as thalidomide, but still.
To put it in perspective, 1 in 33 babies in the US have some form of a birth defect. Meanwhile, no statistically significant increase in birth defects was recorded with atom bomb survivors. That isn’t to say there isn’t any, only that the effect was too small to say yes or no.
https://www.rerf.or.jp/en/programs/roadmap_e/health_effects-en/geneefx-en/birthdef/
https://onlinelibrary.wiley.com/doi/full/10.1111/cga.70013
That’s the conundrum. When sampling populations exposed to major doses or radiation and fallout from nuclear events, the number of radiation induced malformations actually seem to go down. If we’re assuming the effect does exist and the LNT model is right, the data would suggest it’s so small that it simply vanishes in random background noise.
That in turn suggests that trying to do something about the case will cause more trouble than it solves, which in turn leads to greater damage to the people overall.
I am pretty sure there is not 140g of K40 in the body, that is more than likely the total potassium, with the natural abundance being only about 117 ppm of that.
I agree with you, the % of human body by weight are:
Oxygen (O): 65%
Carbon (C): 18.5%
Hydrogen (H): 9.5%
Nitrogen (N): 3%
Calcium (Ca): 1.5%
Phosphorus (P): 1%
(~99% of the human body is the above 6 elements)
Potassium (K): 0.4%
Sodium (Na): 0.2%
Chlorine (Cl): 0.2%
Magnesium (Mn): 0.1%
Sulfur (S): 0.04%
For a 70 kg adult that would work out at about 280 grams of Potassium in total of which about 0.0117% is K40 so about 0.0003276 g or ~0.000328 micro grams of radioactive Potassium-40
Typo I meant to say: 328 micro grams of radioactive Potassium-40
I think you made a math error. I came up with 0.03276 grams or 32.76 mg… 280*0.000117=0.03276
Why don’t we consume largish amounts of pure K39 salts (while avoiding foodstuffs with naturally-high levels of K which contain the K40)?
If we do this consistently, then the body will naturally excrete the excess K, thereby diluting the K40, and reducing the internal background radiation.
The main reason is that it is just as difficult, time consuming, and expensive, to separate K40 from K41 and K39 as it is to separate U-235 from U-238. There is currently no cheap easy solution to separate isotopes of the same element in massive quantities.
The other thing is that all living plants and animals contain potassium, so you would reduce the source of gamma rays (and beta particles) inside your human body but still be exposed to it from all other sources, and gamma rays have more energy than x-rays. So in effect at amazing financial cost you would not really reduce your exposure to natural sources of back radiation by a significant amount. The human body is amazingly efficient at keeping the level of potassium within the human body nearly constant no matter how much is eaten or drank. And the 380 micro grams is mostly in salt form and distributed evenly throughout the entire human body, where ever blood flows potassium goes. And all excess is excreted rapidly, so there is not a buildup in one area of the body.
sorry ~330 micro grams
Are you saying that when I stand next to another (non-treated) human, I’ll get the same dose as I would have anyway, and why should I bother? I think you need to calculate that, and please observe the inverse square law.
So, the excess is rapidly excreted? To my mind this makes for a rapid dilution mechanism, once I start eating pure K39.
Could you calculate the (chemical) half life of the remaining K40? As you say, the blood will distribute all K efficiently around the body.
Gamma rays have such high energy that they pass right through most matter with no interaction. So the K-40 in the human body generating them means that the vast majority of the cells in your body will have no interaction at all! 330 micro grams is approximately 5.1 x 10^18 atoms of potassium-40 with a half-life of about 1.25 billion years (1.25 x 10^9 years or 39.447 x10^15 seconds) that would be about 65 gamma rays per second generated inside your body. At that level of activity, if they were all interacting with your body I think life on earth would be impossible.
Sorry it is less than 65 there are beta particles or positrons or gamma rays generated by each decay event. I’m not going to bother to lookup the decay probabilities for each, so I’m just going to assume 33% chance for each. So I’m going to say about 22 gamma rays a second.
So these beta particles, and positrons (66% of decays) – they sound quite dodgy. Are you sure they are safe?
In any case, you didn’t dispute that the flushing effect will be quite efficacious. So I’m assuming my project is still a go.
Good luck sourcing pure K39 salts.
“Gamma rays have such high energy that they pass right through most matter with no interaction.”
This is GROSSLY incorrect.
Due to the photoelectric effect being dominant at lower energies, photons in the diagnostic x-ray range do get absorbed faster than higher energy photons (gamma rays), with absorption decreasing toward higher energies.
BUT but past a few hundred keV it levels off, and the deposited dose is more or less constant with gamma energy: It still deposits ionizing energy, and a lot of it. The difference being that higher energy gammas go deeper and get to deposit dose in more matter (or body tissue).
I don’t need luck, I have a cunning plan.
After all, this is hackaday!
Look up Radiation Hormesis.
While this applies to background levels of radiation, not consumed food, it was found background radiation slightly above the current background actually improved lifespans for the experimental animals, over the population.
Some individuals may be more sensitive, and others less, but the idea that a 0 radiation level is best does not fit the evidence.
And I note these studies are practically suppressed nowadays. I read one when I had a summer position at Argonne National Lab between undergrad and grad school.
As I’ve said elsewhere, I have been quite close to a fueled reactor (ZPR-9) and do not glow in the dark, nor do I have superpowers (unfortunately for the latter)
Natural radon levels in many places are the more dangerous radiation related hazard. Very high levels are found in very large areas of the US where uranium ores are more common.
“Approximately 300,000 lung cancer cases worldwide each year are attributable to radon exposure, which accounts for about 15% of all lung cancers.
Radon, a naturally occurring radioactive gas, is the second leading cause of lung cancer after smoking. This estimate is derived from epidemiological studies and meta-analyses, which indicate that radon contributes to 3–20% of global lung cancer cases, with a commonly cited midpoint around 15% depending on regional radon levels and smoking prevalence. For instance, a meta-analysis of 28 case-control studies (covering over 13,000 lung cancer cases) reported radon causing 3–14% of cases, while broader reviews estimate up to 20%, particularly higher among never-smokers (up to 30%). The World Health Organization (WHO) confirms radon as a leading cause, with risks increasing linearly by about 16% per 100 Bq/m³ exposure. Globally, lung cancer accounts for roughly 2.1 million new cases annually (per GLOBOCAN data), making the radon-attributable portion approximately 15% or 300,000 cases.
Radon is dangerous because it’s a radioactive gas that emits alpha particles, which can damage DNA in lung cells when inhaled, increasing the risk of lung cancer. It’s colorless, odorless, and tasteless, so it’s hard to detect without testing. Radon comes from the natural decay of uranium in soil, rock, and water, seeping into homes through cracks and gaps. Long-term exposure, especially in poorly ventilated spaces, is the second leading cause of lung cancer after smoking, responsible for about 21,000 lung cancer deaths annually in the U.S. Smokers exposed to high radon levels face an even higher risk due to synergistic effects.”
Radon is emitted by some rocks like granite. In Scotland, many houses were built from granite and had to have extraction pumps fitted to extract Radon from inside the houses.
It is the radioactive decay products of radon that actually deliver the radiation to your lungs. Isotopes of polonium, lead, and bismuth are produced as charged atoms that stick to dust in the air. Such dust can stay in your lungs and airways, or be swallowed; gas, not so much.
When tv was about watching CRTs, you could cleans the dust off the screen with a towel, and then get a few extra clicks by putting that towel on a Geiger counter.
I live above chalk, so my background is pretty low.
Hence the interest in reducing it further, by eliminating K40.