Sometimes it makes sense to go with plain old batteries and off-the-shelf PVC pipe. That’s the thinking behind [Bertrand Selva]’s clever LoRaTube project.
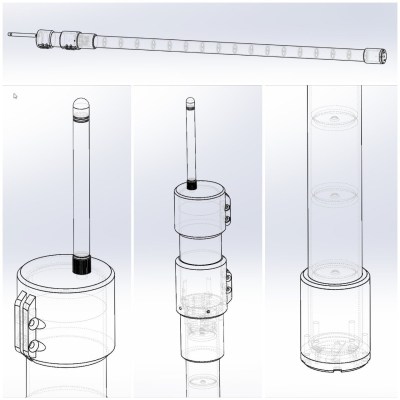
LoRa is a fantastic solution for long-range and low-power wireless communication (and popular, judging by the number of projects built around it) and LoRaTube provides an autonomous repeater, contained entirely in a length of PVC pipe. Out the top comes the antenna and inside is all the necessary hardware, along with a stack of good old D-sized alkaline cells feeding a supercap-buffered power supply of his own design. It’s weatherproof, inexpensive, self-contained, and thanks to extremely low standby current should last a good five years by [Bertrand]’s reckoning.
One can make a quick LoRa repeater in about an hour but while the core hardware can be inexpensive, supporting electronics and components (not to mention enclosure) for off-grid deployment can quickly add significant cost. Solar panels, charge controllers, and a rechargeable power supply also add potential points of failure. Sometimes it makes more sense to go cheap, simple, and rugged. Eighteen D-sized alkaline cells stacked in a PVC tube is as rugged as it is affordable, especially if one gets several years’ worth of operation out of it.
You can watch [Bertrand] raise a LoRaTube repeater and do a range test in the video (French), embedded below. Source code and CAD files are on the project page. Black outdoor helper cat not included.
















Good luck with those D cells lasting 5 years inside the PVC tube.
Can’t speak to their power budget but I’ve had 4x alkaline AAs last for 4 years in similar conditions. No leaks, but I was probably just lucky.
Yeah. I pulled out functioning AAs from low power devices with a “best by” date 25 years in the past.
Never seen an alkaline leak that wasn’t already depleted or damaged by extreme environmental conditions (cooked/crushed). I’m sure it’s possible, but despite a sample size in the low ten thousands, I haven’t seen it before.
What failure mode do you predict? Moisture? Heat? Any engineering trick to ameliorate this in such an application?
Leaking their chemical guts all over the place.
Caused by…? Mine don’t seem to do that unless they run down very low and nobody takes them out for a long time. Ones experiencing constant use don’t seem to, but I could believe that if you stuffed them in a closed pipe and put them in the summer sun for five years they might
Consider yourself lucky.
I’ve lost track of the dollar value of stuff I’ve had destroyed by modern alkaline cells. And no, it’s not because I let ’em die and forgot to replace them for five years. I routinely remove cells if I store something electronic for any length of time.
I’ve literally had new-unused, brand-name cells start leaking while still in the blister pack and still within the “freshness date.”
I had a flashlight with newer cells (still had a bright beam) that I opened to steal the batteries out of (to put into something else)–and found corrosive liquids running out of the tube.
I don’t remember unacceptable behavior 20 or 30 years ago… I think over time manufacturing processes have gotten worse in order to shave off a penny worth of materials here and there and improve profit margins.
Ironically, I’d happily pay MORE for batteries that I knew for certain would not destroy my equipment– which is why I now deploy lithium cells in my expensive test meters, for example.
The leakage issue is overstated. There’s always some that will leak, and some brands that push bad batteries, and then people will conclude that all alkaline cells will leak.
I just took out a 9 Volt battery out of a multimeter, still working but complaining, and the battery was made in 2008. No leaks.
Modern 9v batteries are almost always composed of smaller cells, themselves sealed, as a result they rarely leak. Sometimes this is even stacks of coin cells, as insane as that sounds. That doesn’t make them less terrible as a power source, but it does mean that failure condition is low.
AA and similar packages on there other hand often leak very badly to the extent I’ve considered sealing them separately and it’s not uncommon to see instructions for battery rental during storage.
It’s not overstated, there was a 5+ year period where basically all alkaline batteries leaked.
I had a 20% or higher failure rate from every brand I tried.
Even the stable brands like Energizer, Duracell, Panasonic, and others all leaked their guts out.
Leaking alkaline batteries made up like 85% of my failed electronics for the last decade.
I am STILL dealing with destroyed electronics from years ago that people put batteries in and stuck on a shelf.
It is NOT overstated. It’s rampant. Batteries are now pretty much guaranteed to leak within a couple of years. I’m so sick of having my equipment destroyed by shit batteries.
Funny I didn’t notice anything. When was this?
Like anything, it depends on exactly what subset of batteries you’re looking at. All alkaline batteries everywhere? Yeah, there’s no widespread leakage issue that affects every factory.
Duracells manufactured in Malaysia? Hoo boy, those have been uniformly terrible.
if its Duracell it will puke its guts out within a year.
I’ve had leaky 9-volts kill test equipment before. Anything I’ve bought more recently has had some sort of lithium battery inside it but there is a generation of tool in my shop where I bolted a 9-volt clip to the outside of the case, wired two snap connectors back to back, plugged on into where the battery was meant to be and ran the other outside the case. Then I just keep a couple “currently in use” batteries on the bench and pop them on and off as needed.
But.. I’m not doing that nearly as often now because.. the newer stuff is all built in lithium.
“Modern 9v batteries are almost always composed of smaller cells, themselves sealed…”
I’ve heard this but it wasn’t true for any battery I ever checked. And I have experienced leakage so…. it happens.
Nah. It’ll work fine as-is.
White PVC shouldn’t get too hot even in direct sunlight, and the pipes are liquid-tight by design when properly sealed. The white variety isn’t UV-resistant, but a layer of paint will solve that.
I would place the battery holder in the lowest part of the tube. Then if or when it stops make sure not to tip it in the process of taking it down. This way worst case scenario is having to clean out the leakage and maybe replace the battery holder.
If winter is a thing at this location… How well do D-cells work in the cold? Sounds more like an interesting experiment to try out though, not a reason to not do this. Unless this LoRA link is for something critical…
A friend in middle school (1990s) created an external power supply for a handheld game device (sega lynx) using PVC and D cells, and made one for each of us who had the console.
He called it “The Pipe Bomb,” which he wrote in clear lettering on the thing, just to make it frustrating for us to take through airport security.
This was pre-TSA, so frustrating didn’t mean impossible.
Thanks.
I propose the following as a response to many of the comments received in a new Hackaday log, I outline a revised architecture :
https://hackaday.io/project/203696-loratube/log/245026-response-to-feedback-on-d-cell-lifetime
OK. Given the dogs breakfast of non-metric pipe and tube IDs available in the typical Home Despot, what’s the appropriate type/size/schedule of pipe available here?
Because sure as heck, if you try to buy a 1-3/8 or 1-1/2 inch ID pipe or tube, it’s NOT going to actually be that diameter.
If i buy it by ID or OD, i have always gotten what i want. So, like, if i buy “1-3/8 inch ID pipe”, the ID has always been 1.375 in, obviously plus or minus a few thousandths.
But as you say, that product / labelling by ID is uncommon here. Generally you buy by “nominal size”, which is different from ID (though sometimes they correspond). I don’t know much about all of the standards, CTS vs IPS vs PVC vs CPVC vs PEX vs sch40 etc etc. I do know the standards are not quite the same as common usage. But i have always found it easy in practice to translate between a specific product i can find and what the actual ID/OD are.
My point is, you have to know what your units are. If you buy 1/2″ CTS and come to be surprised that it is not 1/2″ ID, that’s on you. It’s not exactly a secret that CTS and ID are not the same units.
Note that PEX degrades in UV, so if you want to use it make sure to block that.
“It’s not exactly a secret that CTS and ID are not the same units.”
It is new to me? I always thought that units are things like mm, cm, sec or godforbid Inches. I’ve never heard of CTS, ID as in Internal Diameter is a dimension, not a unit.
Is pipe or tube speced by ID or OD?
It depends on the pipe/tube…
No substitute for knowing what you’re doing.
The nominal size thing is pipe that is speced by OD.
Improved material and process means that, while holding ID constant, the OD is now slightly undersize…tapered threads.
These issues don’t stop at pond.
E.g. 4mm ID silicone automotive vacuum tube will be slightly different vs 4mm ID PVC tube.
They are both actually built to securely attach to a 4mm fitting, which isn’t exactly 4mm either.
It’s a nominal size, old school rubber tube was likely actually 4mm ID.
The German car companies ‘fixed’ this…
$150+ (VW)/$400+(Audi/Porsche/Benz/BMW), injection molded vacuum tube fittings.
Each unique, short and brittle, with unique connectors on each end.
To be fair, you can’t assemble them wrong.
But BS, German car mechanics aren’t that stupid, can’t be.
It turns the 10 or 20 year, vacuum tube replacement job into 5k$+.
If you are lucky…
Only have to remove the intake manifold, radiator and accessories to get to the tubes.
Engine out is very possible.
That’s assuming you can wrench.
If you have to pay a mechanic?
Mechanically totaled.
Stupid like foxes…but easy to avoid.
Never buy a water cooled German car!
Wait till you hear about the dimensions of good side of pond 2x4s…
It’s a scandal!
I’m not above walking over to the tool section, grabbing a calliper and carrying that over to the plumbing, lumber or whatever isle. There’s no use guessing or hoping it’s what you need! Just be nice and put the calliper back when you are done.
You go to the store with a D-cell in your pocket. Then you buy the tube where it fits.
If it’s a loose fit, you wrap a piece of cardboard around the cell until it’s no longer rattling around in there.
+1
Haha I was thinking you just stick a wooden dowel down one side of the tube to take up the empty space, but same opinion
My favorite is paper because it’s easier to fold to adjust and get the perfect thickness.
Buy the one that fits? Nah.
For my luck there is something that is a little too big but is cheap. And the one that fits perfect is some sort of high pressure chemical resistant super material that passes every code requirement for carrying flammable gasses in every state. And it’s priced accordingly.
Don’t get me wrong, when safety is an issue, buy the appropriate one. But when all you want is an enclosure I’d rather wrap something around to make it fit or print a holder that fits perfect. A cylinder that just fits the tube you bought with a cylinder subtracted from it that just fits your battery is a pretty easy design!
If you don’t intend to paint it though.. and it’s going to live outside do look for UV resistance though. That is one special feature you do want. Indoor PVC left in the sun year round gets very brittle! But painting it is fine too.
Thanks.
I propose the following as a response to many of the comments received: in a new Hackaday log, I outline a revised architecture :
https://hackaday.io/project/203696-loratube/log/245026-response-to-feedback-on-d-cell-lifetime
Has anybody tried to build something like the mechanism from the clock of the long now foundation with a big weight, a long way for it to drop, and some kind of escapement to power an electronic device for years? I wonder if that would work out well at all..
It’s called a gravity battery, and it’s terrible… You need enormous amounts of mass AND a high elevation to be any useful.
According to https://hypertextbook.com/facts/2001/KhalidaNisimova.shtml , one D battery holds around 81 kJ, and according to the gravity battery calculator, 1 ton suspended on 10 meters have around 100 kJ, so less than 1.5 D batteries.
So for the 18 batteries, and using 50 kJ each to be generous, it would be 900kJ, the equivalent of suspending 9 metric tons 10 meters high, or one metric ton 90 meters high.
I definitely don’t expect it to be ideal, haha. Which is why I’ve never heard of one being used for anything other than incredibly niche applications. I’m more interested in the specifics like you’ve mentioned here…
Although I instinctively doubt that dropping a ton from 90 meters would be the equivalent of 18 D-cell batteries once all factors are accounted for. It doesn’t seem like if you powered a drill with 18 D-cells you’d be able to hoist that ton over 20 stories into the air. Perhaps in the theoretical void without heat or friction. I could be wrong, would be fun to try. Heck, I just want to see the gear train for that.
I looked up some calculations and having 100kg 10 meters high should ideally produce almost 3 watt-hours, not very impressive. Not going to power a radio for very long, not an efficient LoRa or even a good QRP setup. Might get an hour out of it, tops. I can see why nobody does this with electronics, yet somebody who wants to design a gizmo that works for 10,000 years would desire it for its sheer longevity.
LOL. 1 tonne times 10 meters equals 100 kilojoules is literally right in the units. No need to consult an online calculator for that!
Kilograms x 9.8 m/s2 x meters = joules, so you’re almost right except for the acceleration due to gravity at sea level, which is close enough to round to 10. But that isn’t intentionally base-10 like metric units, just a happy accident.
There, you got it, except for the recognition that m/s^2 (acceleration) is just N/kg and a Joule is a N*m.
We were actually very near to have acceleration be 10, because meters were about to be based on 1 second pendulum, but this was abandoned because it depends on local acceleration.
And the definition that replaced it was based on a certain geographical line through Paris, and some arbitrary errors in the math to calculate its length. It was then made into a physical standard, which no-one else could reproduce so the point of varying measurements was made moot.
The pendulum definition could just as well have been standardized to “Greenwich gravity” – or any other place. Then everyone else would have measured their local gravity using the standard length, or any other means to figure out the local correction factor to get the same meter. Everyone can make their own meter standards to an arbitrary degree of accuracy, as long as they agree on the definition of it. The definition is on paper, so you don’t have to carry it around like the physical standard.
So why was one chosen over the other? Well, the pendulum definition was championed by the Dutch and the English scientists who had to deal with a lot of overseas countries and colonies, and the meridian definition was by the French. The revolutionary idea of liberty to the people in France meant of course not the actual people, but the people as represented by the state, or in particular the French revolutionaries who became the state and declared themselves to be the people. A definition of the meter that did not depend on the state, and in particular the French state and their control of the physical prototype, was unthinkable.
I’d like to see it, but I don’t expect t to be all useful. My back of the envelope calculation says that if you have a weight of 1 kg dropping 10 cm a day, you’d only have about 1 J of energy for the whole day. One Watt-second. Approx eleven micro Watt continuously. And for a run time of just a year, you already need a 36 m drop.
As where one of the large alkaline D-cells (~65 g) used in this project are at a minimum 18 kWh. That’s about 65k Watt-second or 65 kJ. Having the same energy for thousands of these mechanical contraptions at a fraction of the cost and complexity.
Same, I wanted to see the attempt more for learning purposes. I assume if it were viable, then somebody would use it, and they clearly don’t… Other than a grandfather clock which you have to wind each morning. Might be interesting with a joule thief as a desk toy or circuit sculpture, an LED clock with a nod to older mechanical clocks using weights and pendulums, something like this.
But probably not any good for any utilitarian purposes except in truly strange and desperate circumstances.
There absolutely is no way hell that a D cell contains 18kWhs. Perhaps a thousand D cells do. My house uses less than that per day. I can’t run my house on a single D cell.
I think you’ve let a “k” in where you shouldn’t.
Of course, and good catch, but it’s not more than a typo. The example still holds, where a single D-cell of 18 Wh holds around 65 kJ of potential energy.
No, the original statement wasn’t meant to imply 18 kWh.
Please have a look at the typical specifications for alkaline D cells.
The usable energy depends strongly on the discharge current.
For low-current applications, a realistic figure is roughly 12 to 21 Ah at about 1.5 V, which gives on the order of 18 to 30 Wh per cell, not kWh.
And for the total pack, energy is rounghly about 0.5kWh :)
Early lighthouse Fresnel lens’s were rotated with a hanging weight and a clockwork mechanism that had a smooth rotation (no jerk like a traditional grandfather clock) the weight had to be cranked up by hand every two to 6 hours depending on the weight of the lens and the height of the lighthouse. Some lens weighed several tons and some lens proper were 8 1/2 ft high or more plus the base structure. Some lens’s were floated in mercury for lower rotational friction.
This is somewhat similar to what you’re asking, I’m sure you will find it interesting but no comments from me on the practicality. He has some newer videos on it too.
https://youtu.be/BSxK5VagSb8?si=UfAliBRFaiqC7a21
When i saw the headline, i thought it was a hack but maybe a bit lackluster — “i built it and it lasted 5 years” is perhaps worth reporting and definitely can be food for thought, but i wouldn’t generally brag about it. Though i guess i have bragged about things i’ve built that have lasted 5 years, when i was so frustrated with expensive mass-produced products that can’t last one year :)
But “i built it and estimated ahead of time that it will last 5 years” is just a report of wishful thinking. I wouldn’t be surprised if it did or if it didn’t but you will definitely learn about its failure modes after time, and only guess about them without time.
It’s not so much that it will last five years, it’s that it will work autonomously in the wild for five years without downtime or maintenance, which is a bit different. But yeah we’ll see how it works out, I suspect it might develop some problems over time too but then again maybe not.
I don’t like plastic. It doesn’t belong into untouched nature, it feels wrong.
And I don’t like LoRa, it’s proprietary. It’s the MS Windows of radio technology.
Where are the open source/open hardware fans when you need them?
Otherwise, nice idea. If it was using wooden pipes/metal pipes/ceramic pipes and something free, such as WSPR or AX.25 (FX.25), I’d like it.
The mono cells used are environmentally fine, if they’re carbon-zinc type.
Though that’s exactly the type that will leak over time (it dissolves throughchemical reaction). ;)
Do you have a preferred alternative to LoRa?
LoRa may be proprietary but it works extremely well for its intended applications. WSPR is a great protocol however assuming this is in the context of amateurs without HAM licenses, ISM bands with duty cycle restrictions will severely restrict transmit intervals for WSPR and many people require faster intervals. AX.25 on the other hand is much faster but the sensitivity of AX.25-capable receivers is much lower than LoRa on comparable receivers.
I feel like a tiny solar panel at the top of the pole and the same capacitors would be way cheaper than that many D cells.
Birds sit and shit on your solar panel. snow falls onto your solar panel, solar panel dies because you’ve bought the cheapest ones or solar panel dies because you’ve bought the wrong one, solar panel dies because you’ve dropped the pole during installation, solar panel does not provide enough energy all year round due to bad power math during the design stage, solar panel catches too much wind and makes the long pole wobble. After 5 years an unattended solar panel will be so dirty it will not perform as intended so you need to compensate for that during the design stage, not impossible, but the real data will be present after 5 years in the field, since you are never fully aware of the local conditions and problems. Did I mention birds?
Batteries are cheap and more predictable.
Perhaps, but the quality of the battery really matters to being predictable longer term, with it would seem a great many folks having huge trouble with leaky crap batteries in these comments.
I’d have to add that Solar panels are also cheap, and with a little care at the design and fitting stage in most places will be self cleaning as weather and gravity work to clean them enough to keep working. Won’t work great under trees, if you get heavy snowfall better be using a vertical panel up a taller stick so it remains clear etc, but for something as low power as this you can cheaply size up the panel enough for it run all year every year easily, and likely for decades if your functional electronics are up to that task (NB to keep it running overnight you are looking at lots of capacitance or a rechargeable battery, and which is likely to be the lifespan limiting point of failure).
I have seen some floating buoys that floated to map ocean currents and other data, some were powered by D cells, – micro processor controlled, no solar panels, the buoy usually was put to sleep for most of the hour, then woke up, data and position packet put together and sent to a satellite, then put back to sleep, depending on the version useful life is one to two years. Some have been built by hobbyists.
GPS is receive-only, you can’t “send data to satellite”.
They probably saved it on SD card and read it later when it came back.
They can send to satellite with a modem, some also use a ham band
Nice example of the KISS principle I think.
I’d prefer some energy harvesting with eg. PV-cells, energy storage and proper energy management – but that gets complicated fast (keep PV-cells clean, bird poop / the whole environment; charge cycles; etc.).
This (principle) is just a drop in “simple” working solution. Probably better / more “efficient” in many situations than ones with energy harvesting.
Thanks.
I propose the following as a response to many of the comments received: in a new Hackaday log, I outline a revised architecture :
https://hackaday.io/project/203696-loratube/log/245026-response-to-feedback-on-d-cell-lifetime
Interesting observations and design, Thanks – Remote anything can be an engineering challenge
The U.S. Coast Guard now uses solar powered Aids to Navigation with rechargeable batteries, LED lights, yes low current draw, the service interval for parts is 5 years, yes they inspect them every few months and the self-contained ones have vertical cells to help keep the cells clean
I would skip the prone to leaking and nowadays pretty difficult to find D cells and go the LiFePO4 route and a (small) solar panel route.
That handles cold well (at least -20°C / -4°F) and would last longer that 5 years
Ive made alkaline recharge fine… And the better the load is matched the better they work, and with a well matched supply emf generator a 5 yr span is likely, whereas the wiring used and flux and dissimilar material and poor quality material will cause corrosion. At some point. In anything. But good copper and brass and non corrosive flux (or bonded connections and no solder,) could last 50yrs. Past that I’m not sure anything from today will work. I think some stuff from the 30s might still work today. But today’s stuff working in 90 yrs? …. Idt so. I see faults rendering it all in need of repair constantly no matter how we collectively try to make it last “forever.” However, “perpetual,” on certain a scale… I believe in.
Yes, I agree.
I own an old BMW E39 530d: the chassis is excellent, the engine as well, but everything in between slowly self-destructs, especially the electronics and wiring, and particularly during winter.
Many of these issues could be avoided with a genuine industrial commitment to designing for longevity: proper material choices, robust connectors, protection against moisture and thermal cycling. And a deliberate search for simplicity as well (nomore can bus in car…). Technically, nothing exotic.
Unfortunately, this is neither sought nor practiced today. Durability is no longer a goal; it is a constraint to be minimized.
So be it. In Europe, in any case, we have largely abandoned industry in the strong sense of the term.