Although metal alloys is not among the most exciting topics for most people, the moment you add the word ‘radioactive’, it does tend to get their attention. So too with the once fairly common Mag-Thor alloys that combine magnesium with thorium, along with other elements, including zinc and aluminium. Its primary use is in aerospace engineering, as these alloys provide useful properties such as heat resistance, high strength and creep resistance that are very welcome in e.g. jet engines.
Most commonly found in the thorium-232 isotope form, there are no stable forms of this element. That said, Th-232 has a half-life of about 14 billion years, making it only very weakly radioactive. Like uranium-238 and uranium-235 it has the unique property of not having stable isotopes and yet still being abundantly around since the formation of the Earth. Thorium is about three times as abundant as uranium and thus rather hard to avoid contact with.
This raises the question of whether thorium alloys are such a big deal, and whether they justify removing something like historical artefacts from museums due to radiation risks, as has happened on a few occasions.
Elemental Facts
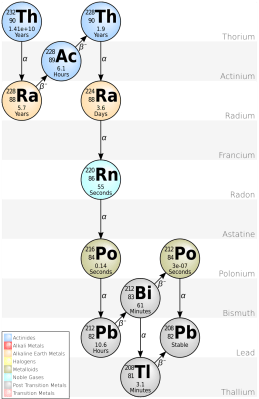
Since the (probably machine-generated) article that inspired these questions didn’t bother to include any useful details or references, it’s time to do a bit of a dive ourselves. This starts with the element thorium and its isotopes.
Obviously the problem with thorium here is not so much with the metal itself or its elementary properties, but rather the fact that a small fraction will decay into radium-228 via alpha decay. This has a half-life measured in years before rapidly passing through actinium-228 to become thorium-228, with a half-life of 1.9 years.
The subsequent decay chain is pretty rapid, taking it through very short-lived isotopes of radon-220, polonium-216 and so on until it becomes stable lead-208. Virtually all of this occurs via alpha decay. Of note is again that the initial isotope here – Th-232 – has a half-life of 14 billion years, or roughly the estimated age of the Universe. This makes it by far the most stable unstable isotope, with U-238 having a half-life of only about 4.463 billion years. Effectively, for most intents and purposes it might as well be a stable isotope.
Thorium is found in most rocks and soil, at around 6 ppm, with several minerals like thorite and monazite containing significantly higher levels.
This raises the question of how dangerous Th-232 truly is, such as when you start concentrating it in some fashion. How much radiation exposure do you experience once you take e.g. thorium ore and wear it around, or concentrate it into pure Th-232 and combine it with magnesium into a metal alloy that people regularly spend time around?
Negative Vibes
One persistent fad in the ‘alternative health’ community is that of negative ions and kin, with many shops selling items like bracelets and similar body-worn items that are supposed to generate these chi-balancing vibes via special ions. Interestingly, some of these are sold with thorium or uranium isotopes embedded in them.
Since these items are worn directly against the skin for extended periods of time, they form an excellent test case of the potential harm of such direct exposure to a significant amount of these isotopes.
According to the fact sheet on on the NRC website, as performed by Oak Ridge National Laboratory (ORNL), these items contain sometimes quite significant quantities of the radioactive material that range from Th-232 to U-238 and even Ra-226, some at more than 0.05% by weight to the point where they would have required a radioactive material license. The estimated local skin equivalent radiation dose was said to be more than the IAEA limit of 50 mSv annually. Despite this, these items require no special disposal methods and you are free to keep using them, albeit with some precautions.
Another study showed an annual exposure of 1.22 mSv, which with the assumed validity of the linear no-threshold (LNT) model would lead one to expect to see some kind of negative health effects. So far these have remained absent despite the popularity of these bracelets and the close contact.
TIG Welding

Outside of accidental exposure in the case of weird bracelets, there is a common use case for thorium, with thoriated tungsten welding electrodes. These are used with DC TIG welding, and contain around 1% (yellow band) to around 2% (red band) of thorium oxide (ThO2). Although an alternative exists with cerium oxide (CeO2) in ceriated tungsten electrodes, thoriated tungsten remains popular due to the long lifespan and good performance with common applications.
Although it’s noted that thoriated tungsten electrodes are radioactive due to the small percentage of thorium within the ThO2, it is such a small amount that no special precautions seem to be warranted. Much like with the thorium oxide found in the aforementioned bracelets and kin, you’ll probably be fine if you don’t try eating it.
Since thorium is also not a heavy metal, unlike uranium, it is in that regard significantly safer, as is its oxide form which does not have the pyrophoric proclivity of the metal form.
Alloys
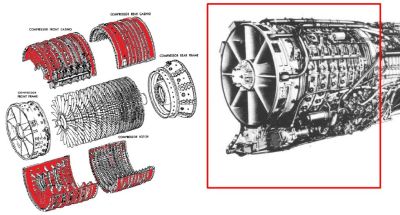
This brings us back to the thorium-metal alloys which started the whole journey. A number of missiles and jet engines have used or currently use Mag-Thor alloys, which has led to for example the Dutch and German defense ministries investigating the radiation exposure from the J-79 jet engines, as found in F-104G Starfighter and F-4 Phantom aircraft.
The reason for this investigation was, as stated, the expected radiation dose when these engines and their respective aircraft are being worked on, handled for disposal, or displayed in a museum or collection. Here we also see the amount of thorium added to the used alloy, at up to 4% by weight, with an average of 1.7%. This means that the overwhelming majority of metal in these alloys is magnesium.
Part of the study was the measured dose at various distances from the components examined, along with a potential cumulative dose. Even in the most conservative scenario the dose came to about 1.2 µSv/hour, or less than 1 mSv/year, since it was probably assumed that people generally do not live 24/7 around these objects.
Realistically, a much bigger potential health risk involving thorium would be something along the lines of incandescent gas lantern mantles, which leads to significant higher exposure to the general public. Not to mention the hazards of the radioactive potassium-40 in something like bananas.

















While no one is likely to try eating a welding electrode, it is common to grind them to sharpen the point, so breathing it is likely if you don’t use proper PPE
Given all the other shit in the air in the average metal fabrication environment I doubt it’s making much difference – if you’re not wearing a mask/respirator you’re breathing in all sorts of dust & fumes.
internal radiation is a lot more dangerous than you think.. especially your exposed and much less protected insides..
1.2 µSv/hour is NOT “…or less than 1 mSv/year….”
It is an order of magnitude greater than 1 mSv/year.
Interesting that you bring up ingestion of Th, but make no mention of the other radioactive isotopes that we ingest every time we put some food into our mouths (or into the mouths of children, which are particularly vulnerable and precious), which no one mentions very much – C14 and K40
I was going to say that this statement: “expect to see some kind of negative health effects. So far these have remained absent despite the popularity of these bracelets and the close contact.”
Makes this article a bit unscientific, because no data was presented to make such a claim.
And is a bit questionable as a (snarky) retort to:
“the (probably machine-generated) article that inspired these questions didn’t bother to include any useful details or reference”
Then I see it’s an article of someone who likes to lobby for the nuclear industry too; so yeah, ok.
‘which with the assumed validity of the linear no-threshold (LNT) model’
The linear no-threshold model is radioactive.
Questioning it can end a scientists career.
If it was true, their would be a statistically significant altitude to ‘negative health effects’ pattern.
To say nothing of geographies that are a little hot.
The population sizes are huge, if this model is correct and effects were linear it should be seen.
Data says: Nope.
Politics says: Shut your scientist mouth.
For clarity, my issue is that there could be many thousands of people that have several health issues, and simply saying that because we didn’t hear about it (nor did we try I expect) everybody is healthy, is just not right.
And that’s assumes there is professional medical intervention and that the medics realize the cause, and if they do register that in some sort of system that researchers can then use to draw inference from.
That 1.22 number being well above the max limit set tell me there is a max limit set, set based on a known statistical risk, and logically there would be health effects.
Oh and that study assumes things like people not wearing the items at night if I recall correctly right? Why assume that? lots of people wear wristbands/jewelry at night, and if they believe for some reason it improves their health it makes it more likely they would.
And the effects don’t even have to be topical to the area of the items, and thus hard to link to the source.
It’s more of a guessed statistical risk, since the statistical probabilities you can measure start to disappear below 100 mSv, and become completely lost in the general background noise at around 10 mSv.
When we measure the statistical risk of cancers below 10 mSv a year, the results show small factors like 1.2 times the probability with a 95% confidence interval from 0.9 – 1.5 which means the confidence interval also includes 1.0 or “no effect”. Studies find correlations to cancer by testing multiple different kinds until they get one where the confidence interval happens to exclude the null result, then they publish that. Then later studies fail to find the same correlation. Like the Swedish study that found a link between power lines and leukemia: they originally tested for 800 different types or cancer. By rolling the dice enough times they found one match, though it had nothing to do with power lines. It was simply luck of the draw.
That’s the whole controversy about the LNT. The effects become too weak to measure, so it’s impossible to say whether there is any safe threshold or whether the effect is linear, because you have no reliable data. At the same time, if the effects are so weak that they cannot even be measured, one can also question whether you should even care: if the LNT were correct, so what? The risk is obviously miniscule and we tolerate far greater risks otherwise.
There is. Airline cabin crews get exposed to an extra 3 mSv per year on average from cosmic rays, and every five years on the job is associated with a small but statistically significant 5-15% increase in the probability of developing some types of cancer.
Though cosmic rays are much more energetic. Gamma rays and ultra fast particles, and all that.
The LNT model is an oversimplification in so many ways that it’s basically useless even if we can show some supporting evidence. It’s simply something to use when people ask why there are certain limits or rules, because otherwise the authorities would have to admit they just pulled the regulations out of a hat.
Radon Bad.
I tend to agree.
Everyone’s gangsta until they realize there are no old welders.
I work with a lot of old welders at a college welding teaching section, some of them keep going in the job well into their 60s and some have been part-time into their early 80s.
The only one I know of in the last quarter-century to have died from cancer, smoked like the proverbial chimney, always with a hand-rolled durry hanging out of the corner of his mouth.
ah yes the old logic fallacy… survivorship bias…
not even worth the time
He probably died of radiation-induced lung cancer from the Polonium in his tobacco. No one ever mentions this – I wonder why not – it seems like it would be a good reason to stop smoking.
What utter BS – there’s plenty of old welders.
An any ill-health among people who work in metal fabrication is unlikely to be down to TIG electrodes given the amount of other fumes & dust floating round and the sometimes quite lax approach to health & safety in some places.
you keep trying to defend a topic yet cant actually substantiate anything… the number of workers based on age alone tells us its probably killing people and much more than any survivorship bias narrative you want to argue.
It’s the burden of proof on you, making the claims with no evidence.
According to the government which never lies to us, the average age of front line production welders is 39, the average age of all employees in the country is 42, and the average age of everybody working at a company that mostly welds is 52. The average age of aseembly line workers (which includes welders and everything else) is 38, so it seems welders live a little longer than people who don’t weld; maybe its good exercise or people who have unhealthy habits can’t learn to weld etc. Doesn’t seem to be much of a signal in the official government data.
There’s a fair amount of evidence that the LNT model for exposure isn’t particularly accurate. Our bodies are pretty good at adapting to low levels of radiation exposure.
Do alpha particles penetrate the skin now?
on the inside or outside of your body.
Alpha particles generally can’t penetrate the skin, but inside the body it can do serious damage. My big worry would be Depleted Uranium rounds that burn and create a nasty cloud of smoke that can get in your lungs and expose its tissue for years. Also note that exposure from the Thorium chain will be limited by it’s billion year half-life.
From the article: “Of note is again that the initial isotope here – Th-232 – has a half-life of 14 billion years … This makes it by far the most stable unstable isotope.”
Incorrect. Bismuth has a half-life of 2.01×10^19 years, and tellurium-128 has a half-life of 2.25×10^24 years.
Aww had hoped we made more progress with thorium
Been tracking it flr 20 years… cmon China invent something not using white phosphorus pleaaassee.
I think it should be a rule that all low-level radiation doses are expressed in units of bananas to stop the sort of alarmist nonsense people leap to whenever it’s mentioned.
I actually caught one on the engines in question on video a few months ago: https://www.youtube.com/watch?v=LZl4C1WqavA
As someone who works in with one of the largest holdings of radioactive material in the museum world, I can say that your article is misleading. While J-79s are low dose threat, in the museum context these are aging components that oxidize at a faster rate than your standard aircraft components that are made of aluminum. The oxidation poses an inhalation and ingestion hazard that is increased if any restoration, painting, or cleaning takes place.
In addition to the inhalation and ingestion issues above you only mention there are ramjets that contain much higher percentages of thorium. The Marquardt RJ43-MA can reach the dose equivalency of a Xray after an hour of exposure. Some optics containing thorium can reach up to thirty percent by weight.
“Thorium is found in most rocks and soil, at around 6 ppm”
Misread this as “6pm”, and thought that was an unusually specific bit of data, especially given the 14bn year half-life! I think it’ll still be there at 6am the next morning! 😂
Good luck finding any new radioactive incandescent lantern mantles these days. I got my μSv/h from antique stores.
Th welding rods work very well in a cloud chamber.
https://vimeo.com/301505834
Some of us spent our careers hand grinding thor-mag housings with carbide burrs. Then we would go smoke cigarettes covered in the dust.
Thorium has been deposited on steel using a jeweler’s trick:
https://phys.org/news/2025-12-jeweler-generation-nuclear-clocks.html
Can that be done with Americium?
Carlo Rubbia needed that tech for his Mars ship idea—you need only 12kg for criticality:
https://www.secretprojects.co.uk/threads/nuclear-powered-spacecraft.3217/page-8#post-858069
I have a couple of drawers full of camera lenses with thorium in them. I know why they were made with thoriated glass , because adding thorium to the glass, a high refractive index (over 1.6) can be achieved while maintaining a low dispersion. All very fine things, other than the glass turning yellow over time unless exposed to a lot of UV light.
So, what I want to know is why thorium in jet engines? Did I miss that sentence in the article? Actually, magnesium seems like a bad idea™️ for hot combustion adjacent structures although Porsche and VW thought it was a fine thing to make engines from.
I suspect the risk of a thorium artifact isn’t casual contact but even the smallest trash can fire that spreads to the mostly magnesium alloy means dust oxide covering every surface in the museum including all the artifacts making the museum a total loss. Well, display it on a ceramic tray sitting on sand and/or suspend a wood can full of sand over the artifact so if there is a fire the artifact will be buried in sand and probably not contaminate the entire building.