Once again, [Afroman] is here for you, this time breaking down electrolyte and the terminology behind batteries.
Volts and Amps are easy mode, but what about Amp hours? They’re not coulombs per second hours, because that wouldn’t make any sense. An Amp hour is a completely different unit podcast, where a 1Ah battery can supply one amp for one hour, or two amps for 30 minutes, or 500 mA for two hours.
Okay, what if you take two batteries and put them in series? That would double the voltage, but have the same Ah rating as a single cell. Does this mean there is the same amount of energy in two batteries as what is found in a single cell? No, so we need a new unit: the Watt hour. That’s Volts times Amp hours, or more incorrectly, one joule per second hour.
Now it’s a question of the number of cells in a battery. What’s the terminology for the number of cells? S. If there are three cells in a battery, that battery has a 3S rating. You would think that C would be the best letter of the alphabet to use for this metric, but C is entirely different. Nothing here makes any sense at all.
What is C? That’s related to the number of amps a battery can discharge safely. If a 20C battery can discharge 2200mAh, it can deliver a maximum current of 44 A, with 20C times 2.2Ah being 44A.
So there you go. A complete description of something you can’t use logic and inference to reason through. Video below.















“What is C? That’s related to the number of amps a battery can discharge safely. If a 20C battery can discharge 2200mAh, it can deliver a maximum current of 44 A, with 20C times 2.2Ah being 44A.”
The reason it doesn’t make sense to you is because you’ve got it wrong.
You confuse power and energy. Straighten that out and you’ll feel better.
Brian – There’s nothing at all wrong writing amp-hours as (coulomb/second)-hours. In fact, cancelling the two time units (1 hour/second = 3600) gives us the insight that an amp-hour (current over time) is perhaps more fundamentally a measure of charge, 3600 coulombs, one coulomb being roughly equivalent to 6,200,000,000,000,000,000 electrons (or other unit charge carriers).
Similarly, while watts measure power, watt-hours give energy. In this case, 1 watt-hour = 1 (joule/sec)-hour = 1 joule*(hour/sec) = 3600 joules of energy. Watt-hours are useful in electronics, but a joules are the standard SI unit for energy.
Unexpected unit combinations crop up all the time when working in physics. At the end of your calculation, cancelling and rewriting units can often provide a useful kind of check on your work.
It’s also useful during exams :-) there were a few occasions when I found it easier to memorize the units and derive the equation for something based on them rather than memorize the equation.
It’s also good fun to define things in odd units like barleycorns.
Ken, you explained the units a lot more diplomatically than I was going to :). Not really that complicated really.
This type of unit calculation shouldn’t be at all unfamiliar to anyone who’s been taught proper use of S.I. Kilogram metres squared per second squared, anyone? It should be totally straightforward that if you have a quantity like power (energy/time) and multiply it by a time you get energy. Measuring speed in hertz per dioptre is a silly trick but even so, you should be able to understand how it translates to m/s.
Also complaining about the S terminology used to describe the number of cells in parallel (when they would apparently prefer C, presumably for “cells”) is extremely shortsighted. It’s 3S to distinguish from 3P, 3 cells in Parallel.
The C rating for discharge is something I’m not so happy about because it makes a numerical comparison between two different quantities (amps and amp-hours) so the C rating implicitly contains a unit of 1/hours. If you are going to provide two numbers (the capacity and C rating), why not instead provide the capacity and max discharge current?
The units for C actually DO make sense. 1/hours is the same as saying “per hour”, so the C rate tells how many times you would charge (or discharge) a battery per hour. The advantage is that as long as you’re comparing apples to apples – batteries of the same chemistry – characterizing a cell at different C rates makes it simple to adjust for different battery sizes. That is, if you have “AA”, “C”, and “D” batteries from the same manufacturer using the same chemistry, they will behave similarly at a given C rate. This used to be how battery manufacturers recommended charging NiCd batteries; in ancient times (ca. 1970) it was typical for a manufacturer to use the same data sheet to cover a number of sizes of cells, and just specify that they should be charged at, for example, the 10-hour rate, or 1/10 C, and either way this meant for example that a 1.5 A*hr cell should be charged at 0.15 A for 10 hours, rather than spelling out the charging current for each cell size separately.
Yeah, we should use ‘C’ for the number of Cells in series. We also need to describe the number of cells in parallel. but that’s also a number of Cells, so we’ll use ‘C’ for that, too. Then we can talk about a 6-cell battery being connected as 2C3C or 3C2C, and know what voltage it is with no ambiguity!
Or maybe we should go on using S for series and P for parallel, and punch the face of anyone who calls it “maddening”.
S stands for ‘Series’ and P stands for ‘Parallel’ … so technical a 3 cell battery is “3S1P” and a battery with 6 cells could either be “3S2P” (so that’s 2x in parallel, with 3x of these put together) or as “2S3P” (so that’s 3x in parallel, with 2x of these put together). With the S and P you can easily know how many of the pack are in series and how many in parallel, and multiplying S by P will give you the TOTAL number of cells
oh btw S and P are already the terms used (at least in the RC world)
Yes, that was exactly my point — apparently I should’ve used sarcasm tags…
There are cells and there are batteries, which are comprised of two or more cells. There is no such thing as a single cell battery. A cell is just a cell.
AAAA, AAA, AA, C and D cells, not batteries. A 9V or 6V is a battery because they have more than one cell. Most 6V lantern batteries have four cylindrical cells inside. Some 9V batteries have 6 AAAA cells that can be removed and used in the few devices that use that size cell. Removing the metal housing can save a gram or so for applications where every bit of weight savings is needed.
The other common 9V design packs six flat, rectangular cells into a plastic shrinkwrap.
What can cause some confusion is that carbon-zinc cells and batteries are commonly called “heavy duty” or even “super heavy duty” when their performance is anything but! They are not at all suitable for high drain devices.
Along with alkaline cells and batteries, carbon-zinc produces 1.2V per cell. Nickel-cadmium and nickle-metal-hydride produce 1.2V per cell. I used to have a portable CB radio that ran on either eight alkaline AA cells plus two dummy cells or ten rechargeables. It required 12 volts but not one bit more. Someone had loaded it up with ten alkalines and the power circuitry did not like being fed 15V!
Complicating matters further, lead-acid chemistry produces 2V per cell, thus a 6V LA battery needs only three cells.
I’d like to see lithium-ion cells packaged in the common AAA through D cell sizes, with built in regulating circuitry to control the output to 1.5 or 1.2 volts. They’d require special contacts and chargers and charging outside devices. Something like how the Renewal rechargeable alkaline cells worked in their original, Renewal only chargers. BTW, the second version Renewal chargers also work with NiMH and NiCd with individual cell charging so all get brought to the same charge level.
Last I checked on eBay there was a ton of the first version (totally useless since the cells are long discontinued) Renewal chargers.
How about a hack to convert those doorstops into useful chargers?
66
There is no such thing as a single cell battery. A cell is just a cell.
99
A single-cell battery is just a set that contains only one element. I find that perfectly valid.
True. If you wish, you can define a ‘battery’ as “one or more cells connected in series”. I don’t know why people have to be so pedantic about this.
It’s the same people who flip out when someone uses “they” as a gender-neutral singular pronoun.
Carbon cells with zinc chloride electrolyte are “super heavy duty” in comparison to the original (ammonium chloride) carbon zinc they replaced (which no longer exist). The name stuck, despite better technology coming along. I guess they should have named alkaline cells “super super heavy duty”.
Freshman year we did a rough design of turbine generators and calculated the output with certain conditions, I used the term ‘watt hours per hour’… caffeine and minimalism do not mix well in engineering.
I’ve done in the past a little overview like this (but in text) for RC Lipo’s http://www.rcgroups.com/forums/showthread.php?t=1373776
And let’s not forget a 1Ah gel-cell battery can supply 0.1A for 10 hours, or 1A for maybe 10 minutes. (Charge mobility in the gelled electrolyte is low. If you discharge one quickly, you can completely drain the charge from the gel immediately surrounding the electrodes, making the battery act as if it were dead… yet disconnect it, and it will miraculously “recharge” itself over time as the charge redistributes in the gel.)
It’s not fair to say logic and inference won’t serve you. It all makes perfect sense, it just requires some effort to grasp. Especially if you’re trying to familiarize yourself with multiple chemistries simultaneously.
Ah! Something maddeningly hard to explain to non-engineers, simply and plainly explained.
http anyone
http://www.youtube.com/watch?v=cxkVxi9P0EA
The video is more than decent even if very basic. Why did you have to write such an awful article to introduce it? I only watched it because I had to click “read more” to come here comment about how bad the text is…
Anybody feel like they want to support Afroman in making more of these excellent videos just needs to go here http://www.patreon.com/afrotechmods
“what about Amp hours? They’re not coulombs per second hours, because that wouldn’t make any sense.”
Sure it does, you’re just writing it wrong. A better way of expressing it would probably be “(coulombs per second) hours” and if you do the simple conversion of 3600 seconds/hour and cancel out the time dependencies, you’ll get coulombs. Coulombs are a measure of charge, amp-hours are a measure of charge. Coulombs are a bit of an inaccessible unit, though, so the industry has settled on amp-hours.
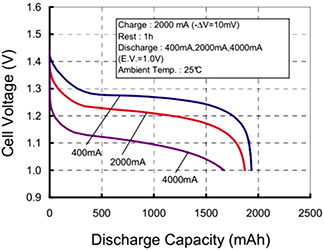
What doesn’t make sense, and is in fact incorrect, is “…a 1Ah battery can supply one amp for one hour, or two amps for 30 minutes, or 500 mA for two hours.” This is actually contradicted by the (more accurate) graph immediately next to the sentence. A battery will always have its rated capacity specified at some rate of discharge, that rate often being dependent on battery chemistry and intended service. It will always be worse for higher rates of discharge and almost always be better for lower rates, depending on chemistry, construction, environment, and other factors.
And again, “…we need a new unit: the Watt hour. That’s Volts times Amp hours, or more incorrectly, one joule per second hour.”
What’s incorrect here? Again, cancel the time units if they confuse you. You end up with joules, a unit of energy. Watt-hours are also a unit of energy. All of this is high school level physics at best.
good, I dont ahve to say anything about S and P…..
You cant get 1 amp for 1 hour from a 1Ah battery, its the hypothetical down to 0V, which you cant do, AND its typically the 20hr rate, which means the constant current at which its done after 20 hours,
Batteries have internal resistance loss, so the more you load them over that ’20hr’ rate (current), the more power they lose internally, aka, the less power you get out of them.
AND then define ‘done’…
For a lead acid battery, your ‘done’ at 80%, and hey, thats a long ways from 0V…..
The best thing to do is look at the datasheet for the, or a similar battery, put your estimated current against it and get an approximation of runtime.
These things hold true (mostly):
– double the load halves the runtime (with exception above)
– double the capacity doubles the runtime
Watt-hours are amp*hours * V, it can be a usefull term when trying to describe how much actual power a bank can hold. being that, as mentioned above, amp-hours dosn’t say much about a battery.
Everyone understand why a lithium cell has 4.2/1.5 of the watt-hour rating of a alkaline of the same Ah rating?
Shall I post the information for my battery discharge chart plotter thing a ma jigger to the tips line?
I’m pretty sure that capacities are calculated for the use full voltage range, not from the maximum voltage to zero. Otherwise there would be little point referring to it. On the other hand less than scrupulous manufacturers might do this to exaggerate capacity :)
The units for C are 1/hr, actually, or inverse hours. Amp-hr ratings are meaningless in the absence of the specific test conditions – for automotive deep cells, that specific test condition is a twenty hour period. A 100 ah rated auto battery will,supply 100 ah / 20 h = 5 a before the voltage drops to around 10.5 volts. Lithium mfg’s don’t typically tell you the test condition time….