Researchers in Singapore have created a new kind of redox flow battery with an energy density around ten times higher than conventional redox flow batteries. Never heard of a redox flow battery? These rechargeable batteries have more in common with fuel cells than conventional batteries. They use two circulating liquids separated by a membrane as an electrolyte. Each liquid has its own tank, and you can recharge it by pumping in fresh electrolyte. The redox in the name is short for reduction-oxidation and refers to the process that stores energy in the two liquids. You can learn more about flow batteries in the video from Harvard below.
The energy capacity of a redox flow battery depends on the volume of the liquid tanks. This property makes them attractive for storing lots of energy (for example, the output of a solar or wind generator). What makes traditional cells unattractive is that they have low specific energy — making them too heavy for something like electric vehicles — and low specific power, which makes them expensive for storing energy from stationary sources like wind turbines. The new cells, using a lithium-based solution on the cathode and a titanium-based solution on the anode, allows for cells that store about 500 watt-hours per liter of solution.
We’ve talked about homebrew molten salt batteries and lithium-air batteries, too. Building a redox flow battery doesn’t seem that difficult if you can master the membrane. Typical materials include glass-ceramics and Nafion polymer. The new battery uses a composite membrane made from Nafion and PVDF.














So that’s almost twice the density of available LiPo (before the storage tanks etc)
So, Using the same tanks and the fact that electron flow is reversed from charging to using, then you would have to use two membrane flows from the same tanks in order to both use and recharge at the same time, right? Because last I checked wind doesn’t stop blowing just because the sun go down..
Or you could use an off-the-shelf power controller and use it to charge the redox battery when supply exceeds demand and discharge it when demand exceeds supply.
From the /. summary: “But he adds that even though the novel battery has a high energy density, the rate at which it delivers that power is 10,000 times slower than conventional flow batteries, far too slow for most applications. Wang and his colleagues acknowledge the limitation, but they say they should be able to improve the delivery rate with further improvements to the membrane and the charge-ferrying redox mediators.”
So still useless for an electric vehicle. TANSTAAFL
Perhaps, but it would be interesting to know how surface area of the membrane changes the charge/discharge rate.
Simple batteries could not put out enough current for an electric car either until they were improved on.
There are membraneless designs that work on laminar flow.
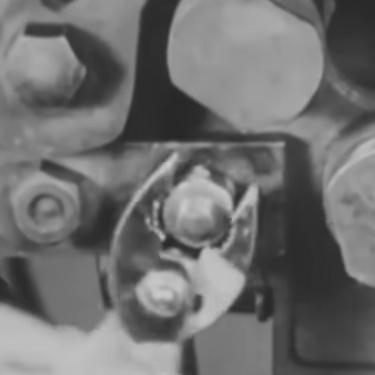
The main problem here is that the tanks aren’t exactly liquid. They’re a solid matrix of the active materials.
The structure is conceptually the same as in a conventional battery, but instead of having an anode-separator-cathode sandwich that is soaked in electrolyte, they have pulled the sandwich apart into two containers and circulate the electrolyte to and from the separator membrane.
That’s what makes it slow. Here, the liquid is carrying dissolved ions between the membrane and the active material. In regular redox-flow batteries, all the active material is dissolved in the electrolyte, so it comes to direct contact with the membrane as in the conventional battery.
As you can see from the picture, the active material is a slurry that sits at the bottom of the tank. It doesn’t mix very well with the electrolyte which makes the ion exchange more difficult, and it would be difficult to pump around the system because it contains solid particles that cause abrasion.
The EV problem isn’t the battery in any case. It’s not energy storage that’s the problem, it’s energy delivery.
If you do the math, a gasoline pump filling up a petromobile is something like 6-10 MW of power.
Unless EVSEs come with a Mr. Fusion, that’s not going to be happening.
Just another weekly revolutionary game changing battery technology.
“Building a redox flow battery doesn’t seem that difficult if you can master the membrane.”
Always those pesky membranes holding things back. :)
Well, maybe one other thing. The cool thing about redox flow batteries is how easy and cheap it is, at least theoretically, to scale up the capacity. But suppose you were to actually do that, and use it as the primary storage for your off-grid solar system. Now what if you have an earthquake/fire/tornado/etc, that causes the solutions to spill into your home and yard? The solutions I’ve seen used so far are quite toxic. This would be a professional decontamination job, costing major dollars, and maybe some government/EPA fines as well. It’s rather scary if you think about it.
There are organic redox-flow chemistries that use stuff like quinone, which isn’t particularily toxic or caustic, and in fact has the problem that you have to be hygienic in handling it because bacteria would like to eat it.
Cool, I didn’t know about that. Though in the example I looked up the electrolyte is nasty instead, requiring either hydrobromic acid or ferrocyanide; neither of which would be pleasant in an uncontrolled spill.
I guess nothing’s perfect, but if there were a version of this technology using liquid components that are cheap and fairly non-toxic, it might have hacker applications even if the energy density is too low for commercial interest. Kind of like how some solar energy enthusiasts still use NiFe batteries.
how is this any different then your heating oil tank rupturing? seems like exactly the same scenario to me except your not using fossil fuel using this setup
Fancy. I still think the good old pumped-storage plants would be a much cleaner and safer way to store MASSIVE amounts of power. The problem we have in switzerland where there are quite a few of these plants and the topology in the alps would be ideal, we still have too many “environmentalists” that would try to block any new projects to make more massive dams and storage lakes. As if using very expensive high tech and extremely toxic chemicals on a massive scale just to be a little bit more efficient would be the better solution…
The energy density of pumped hydro storage is unfortunately massively bad. There isn’t enough suitable places, and they become huge flood hazards to the low-lying lands once they’re a 100 years old.
If I remember correctly, if all of Norway was dammed up, there would be something like two days worth of power reserve for Europe.
The gas grid in Europe already holds enough gas for two months. To me the solution is simple: learn how to make more gas.
the point of a pumped hydro storage in the case of switzerland is not long term storage more like a huge buffer to even out demand spikes and the advantage here is you can run it backwards meaning you can “recharge” it when demand is low like during the night. and all you need is power. you can store gas and use it to even out spikes as well but you cant run the gas grid backwards.
It is a very location based solution. It would be pretty useless here in Florida.
In other words you are correct for your location.
Pumped hydro is common place here in scotland, it also rains enough I imagine it goes a fair way to offset pumping losses, and no concern about toxic leaks.
We don’t need this, “Barrerizer” is coming!
Sorry, my spell-checker meant “Batterizer”, it’s drunk (again).
“And in this way, the battery provides almost all of the power to operate the 2 small electric pumps, here…”
[sorry, someone had to make the cheap joke]