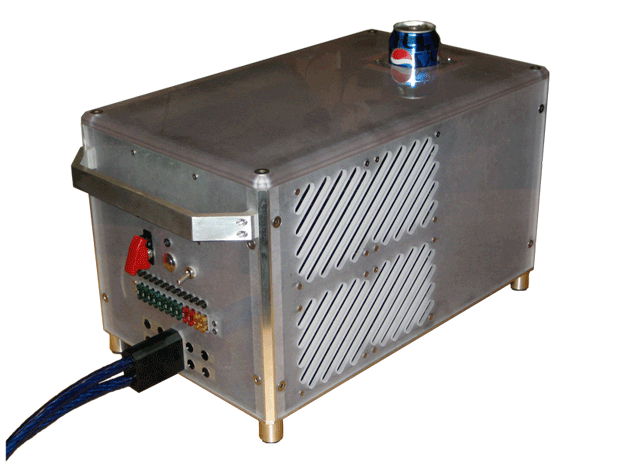
We’ve covered many thermoelectric beverage coolers in the past, but none come close to the insane power of the AbsolutZero. [Ilan Moyer] set out to design a beverage cooler that chills a drink from room temperature to 5 degrees Celsius as quickly as possible, and it looks like he succeeded. The AbsolutZero consumes around 2.5kW of power and runs 8 water-cooled thermoelectric modules to quickly chill a drink.
[Ilan] put his machinist skills to work and fabricated many custom parts for this build. He machined water blocks for each thermoelectric cooler out of solid copper which draw heat away from each thermoelectric cooler. He also fabricated his own bus bars to handle the 200A+ of current the system draws. To transfer heat from the beverage to the thermoelectric modules, he turned and milled a heat spreader that perfectly fits a can of any beverage.
[Ilan]’s design uses a closed-loop water cooling system and 4 radiators to dissipate all of the heat the system produces, which is quite a lot: thermoelectric modules are typically only 10-15% efficient. The whole design is buttoned up in a custom polycarbonate enclosure with a carrying handle so you can conveniently lug the massive setup wherever quickly chilled beverages are needed. Be sure to check out [Ilan]’s build photos to see his excellent machining work.
Thanks for the tip, [Stefan].















Awesome build, but am curious as to how quick ‘as quickly as possible’ actually is…?
Ah, just read the front page..Under two minutes. Noice.
Noice!
no ice
Highly inefficient and not as impressive as this thing which cools cans to 3C in 2 minutes…
https://www.youtube.com/watch?v=VN-h5XkUyF8
and yes, I know it’s not impressive in its complexity or what-have-you but rather its simplicity.
Working construction we used to cool beers all the time by just spinning them in a bucket of ice like the above machine. After about a minute the ice forms into a perfect beer shape and you can spin it pretty quickly. Takes around 2 minutes for an ice cold beer and surprisingly little foam. Great example of overkill and a fun project! I bet this was fun to make.
The reason you get little foam is that gas (CO2) solubility is higher in cooler liquids (water).
Add salt to the water and you will see a increase in efficiency.
“Just before the engine switches to rocket mode at Mach 5, the incoming air will have to be cooled from 1,832 degrees Fahrenheit (1,000 degrees Celsius) to minus 238 degrees Fahrenheit (minus 150 degrees C), in one one-hundredth of a second, displacing 400 megawatts of heat energy using technology that weighs less than 2756 pounds (1,250 kg).”
http://www.space.com/22004-skylon-space-plane-rocket-engine.html
Yes… That might be quick enough for my beer.
Same power budget with a set of stirling coolers and you get some liquid nitrogen in no time ! :)
Beat the Peltier modules by far.
THIS!
How much does a Stirling cooler cost? (Something that can get down to -60C, even better if it can go lower) Sounds like just the thing for a project of mine!
Believe it or not, Coleman sold very good Stirling coolers for ~$300 for a while and apparently they worked great – nobody bought them because who spends $300 on something that looks like a small ice chest?
Artifact listing: http://amzn.to/1u2QVVX
Does seem odd to go to all that trouble to use peltier modules which are pretty inefficient. Unless he just had them kicking about it’s an odd choice.
Peltiers are relatively cheap, available, and easy to use due to their solid state nature. So despite their known inefficiency, it’s still attractive to try and make it work.
Mythbusters did an episode about this once. Salt and ice proved to be the quickest and most efficient way to cool a beverage using chemistry. A C02 fire extinguisher was the fastest, but wasn’t efficient. The salt and ice can be used to cool several cans or bottles to the desired temperature in under 3 minutes. The only power draw being what was used to create the ice.
So make a deep fryer style basket to lower a drink into a tank of ice water and salt. With a blender mixing / increasing the surface area of the ice to come in contact with the salt and you could probably make 2 minutes.
After thinking about this off and on, I now believe that a planned and built project would be to use an old dehumidifier. If half of the coils are separated and sunk into a coolant solution, such as alcohol, the active compressor on the dehumidifier would cool the liquid to below freezing. Cans or bottles lowered into the coolant solution tube around the submerged coils would be cooled fairly quickly, especially if a current was created to keep the cooled liquid moving over the containers and the cooling coils.
I’ve even considered cooling a desktop PC processor and graphics card in a similar fashion by pumping the coolant for the PC through the coolant tank of the dehumidifier.
The downside is the compressor uses and requires electricity while the ice and salt do not. This would be for home use or where there was a source of power.
Quite old “news”: It received MIT’s 2007 deFlorez Award
Very old indeed.. From the author’s “portfolio” :
AbsolutZero Rapid Beverage Chiller
January 2007
Love the overkill, kick back and enjoy a cold one.
Just coil some copper tubing around a can and hook it up to a hacked window A/C unit. Many years back, someone actually built an insanely fast ice maker (something like 3 minutes to make a tray of ice cubes!) using parts out of a window A/C.
Part of the challenge was to use electricity as the only consumable resource. Refrigerants in A/C units do not last forever and so I would think it would be considered a consumable.
Window A/C units (and refrigerators) are sealed systems. The Refrigerant lasts until you open the system recover the Refrigerant for recycling, to make a major repair (unlikely on a cheap window A/C) or dispose of the unit.
You’re probably thinking of a car system that ends up leaking due to the use of rubber hoses and the compressor shaft extending outside of the “sealed” system.
Considering I’m looking at a 30 year old freezer right now (never recharged), I’d say refrigerant isn’t a consumable.
My grandparents had an old Amana upright freezer. The walls on it were at least 6 inches thick and the door was like a vault door. It was in the basement when they bought their house, which they lived in for over 50 years. The freezer was only off when there was a power outage.
Any “modern” freezers going to last around 60 years?
I had a freezer that an aunt and uncle bought new in 1971. My family had it since the early 90’s. We donated it to a church where it’s still going. 43 years.
As a former PC cooling nut I kinda shuddered when I saw his water block designs. Those serpentine designs are really pre 2002 era. Then he goes and mills the fins out of it to save time thus reducing cooling ability. Even though he redesigns the blocks gaskets because of galvanic corrosion, it’s still not going to protect it. He should have used full copper in the first place.
However I do love the absolutely insane nature of the project.
As someone who works with waterblocks for industrial electronics, I shuddered a little too — the boundary layer issues mean that the increased surface area of those serpentines (as I’m sure you know) means that they don’t really get you any more surface area! All about turbulent flow.
I would have had the peltiers directly fan-cooled and then had them chill a recirculated brine or propylene glycol mix that was in direct contact with the can.
As a person how doesn’t work in an area that has anything to do with cooling / thermal transfer, I would have completely skipped the water cooling and use ionized air over heat sink like structures.
In the end the cooling power of this unit is determined by the air cooling (in conjunction with the limits of the peltiers) so that is where there is room for improvement. This unit has high secondary thermal re-absorption.
This article cover one of the two aspects of the thermal transfer efficiency of air flow based systems –
http://www.electronics-cooling.com/2012/03/ionic-winds-a-new-frontier-for-air-cooling/
The other aspect is thermal re-absorption – heat is the increase in the the speed of electrons that is transferred photonically to the secondary environment. There is very little to prevent the secondary environment from returning the heat back to the primary environment (emitter) by the same method and this strongly influences thermal transfer efficiency. That is why heat sinks are iodized etc.
Nup, would still be best with current tech to use fans with radiators and use a second loop like jtl3 said. Having the TEMs water cooled allows you to move heat away from the hot side faster than with a HSF. Except for the crazy heatpipe based CPU HSFs, the radiators have a much higher surface area for the heat to escape through. In this setup they would have to be high flow low pressure drop ones to be able to counter the lack of cooling of the water blocks. I could go on all day about the issues with his loop and blocks.
Oh, and heatsinks are not Iodized, they are Anodized. They usually are only on amplifiers and industrial components where corrosion protection is needed. Anodizing increases the thickness of the Aluminium Oxide layer on the surface of the heatsink and adds a dye to it for colour. Raw Aluminium dissipates heat better.
In reply to Jehu, Anodized heatsinks are actually much more efficient heatsinks. Then anodizing improves the emissivity of an aluminium heatsink by an order of magnitude or more. All up this typically adds to an approximately 10% increase in cooling ability.
Mike T. Ok, I stand corrected. However that is really only true in the case of passive, convection cooled sinks with a large fin gap. For most PC HSF where you have a lot of thin fins with small spaces between them for a high surface area and forced air flow, bare metal isn’t that much less and it doesn’t justify the added cost of anodizing.
Seems really pointless to spend that much cost and power on Peltier elements, compared to a small vapor-compression system with a custom-shaped evaporator, the sort of thing that PC cooling/overclocking wonks have been doing for many years.
I wonder how much time could be reduced if the can was shaken or swirled.
But then its more likely to foam over when you open it
Just want to point out that this project was as much my friend Greg Schroll’s as it was mine. We were both equally involved in its design and fabrication. He’s made a bunch of even cooler (:-) things including a gyroscopically propelled spherical robot : http://www.carbiderobotics.com/about/